PSYC 304 Midterm 3: Things To Know
1/79
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
80 Terms
which method would choose to study: loss of grey matter in the weeks following a stroke?
CT scans can visualize loss of grey matter well, MRI is another option
which method would choose to study: changes in non-cortical brain activity following a stroke?
fMRI can see how BOLD response in specific regions changes or PET can visualize loss of function
which method would choose to study: cortical activity while running on a treadmill?
EEG - specialized to record electrical activity in motion
which method would choose to study: the external stimuli and situations in which a neuron fires
single-cell recording, extracellular or intracellular single-unit - what goes on outside a neuron or what the neuron’s path look like
which method would choose to study: changes in protein expression following a neurodegenerative disease (ex. Alzheimer’s)
PET - can see specific intensity of proteins in each cortical area
which method would choose to study: the role of the hippocampus in spatial learning and navigation
lesion studies + Morris Water Maze - designed to measure hippocampus’s role in spatial learning
which method would choose to study: changes in decision making and motivation following acute and chronic drug use
event-related fMRI (humans), maze task (rats) - over many trials, follow progression of drug effects
which method would choose to study: how we select words from our vocabulary for speaking?
fMRI - good for identifying specific regions
which method would choose to study: the role of monoamine neurotransmitters in the motivation?
PET - track neurotransmitter systems, selective chemical lesions (6-hydroxydopamine) and reversible lesions (rats)
which method would choose to study: what regions of the motor cortex control what parts of the body?
TMS - lesions can take away function of region temporarily
what are X-Rays good for?
assessing skull fractures/structural damage
imaging foreign objects in the brain
best for bone resolution
what are CT scans good for?
imagining white matter neurodegenerative (schizo, psychopathy)
tumour/hemorrhage detection (sensitive to blood/calcification)
bone injuries
what are MRIs good for?
soft tissue abnormalities (tumours)
neurodegenerative diseases
stroke detection
detailed brain imaging
what are PETs good for?
imaging progressing of a drug or neurotransmitter system
metabolic changes
what are DTIs good for?
white matter diseases
what are fMRIs good for?
studying brain activity during tasks in specific brain areas
brain during resting states
whole brain studies
what is TMS good for?
establishing causality
mapping functional brain activity
neuroplasticity (rTMS)
temporal precision
what is ERP good for?
temporal resolution
isolation of cognitive components
changes in behaviour
responses to certain stimuli
what causes BOLD?
activity in astrocytes due to synaptic transmission signals opening CA channels which causes blood vessels to dilate and release more blood flow in the brain area - why there is a delay in BOLD response after stimulation (takes a bit longer for this process to happen)
default mode network: what brain regions show significant activity during rest?
medial PFC, posterior parietal cortex, hippocampus, lateral temporal cortex
when is paired image subtraction useful?
functional brain imaging - since we want to minimize randomness when determining activity localization
fMRI
PET
problems with interpreting fMRI studies
spatial averaging: average actually doesn’t represent anyone
spatial resolution: million neurons/voxel
temporal resolution: delay
not necessarily a necessity
focus on increases in activity: some areas of brain are more active at rest (rsfcMRI and default mode network)
regional hemodynamics: BOLD response varies
anxiety and boredom confounds
drugs (caffeine, nicotine, medications)
anticipatory hemodynamics: BOLD response when we expect a stimulus
low between trials (30-40%)
statistics (0.05 error rate = high # due to amount of fMRI studies)
how do MRIs work?
each hydrogen atom rotates randomly about its axis
when placed in a magnetic field, they will align according to their north or south poles
a radio frequency pulse knocks atoms out of alignment, but still in same magnetic field - makes them want to relax back to orientation
energy that produces magnetic fields is released as atoms try to go back to original orientation
intravenous drug injection
injections into veins through catheters
drug self-administration studies
cocaine, heroin
likely to knock out catheters
intramuscular drug injection
injections into muscles - enters blood stream quickly
can leave a lot of soreness
subcutaneous drug injection
just under the skin - rats hardly feel it because of their flappy skin
takes longer to get into bloodstream - ideal for medications that need to be slowly absorbed (insulin)
intraperitoneal drug injection
into abdominal cavity - quickly gets into blood
most common - rats don’t really feel it
intraventricular drug injections
injections into ventricles
overcomes blood-brain barrier
chemotherapy
nissl staining
captures density of neurons
golgi staining
captures individual neurons
optogenetics
controlling and observing activity of neurons using light
light-sensitive proteins (channelrhodopsins) are inserted into DNA of specific neurons
fibre optic cable is implanted into brain, targeting area of interest
light delivered through fibre optic cable
allows for precise control over specific brain regions, detailed studies of neural circuits
top-down processing
formulate hypothesis of nature of stimulus → select and examine stimulus to check hypothesis → recognize stimulus
bottom-up processing
detect specific features of stimulus → combine specific features into complex forms → recognize stimulus (how we think in class)
how do we determine what system neural information reflection?
doctrine of specific nerve energies: specialized cells are sensitive to only energies they are fitted for (some exceptions)
labelled lines theory: specialized cells stay segregated from other types of sensory info
what are the steps of touch processing?
receptor detects touch stimulation
stimulation of the receptor stretches the tip of the axon
produce a graded potential with an amplitude directly proportional to strength of stimulus, opens gated ion channels in membrane
when the receptor potential is big enough, an AP is generated
transduction
turning external energy into nervous system signals
ionotropic receptors (synaptic transmission)
metabotropic (GCPRs)
how are different intensities of a stimulus represented in nervous system?
more intense stimuli generate more rapid APs OR more neurons fire parallel to each other
range fractionation
a wide range of intensity values can be coded by a group of cells each of which is a specialist for a particular range of intensities
Meissner’s Corpuscle
light touch - in the dermis, small receptive fields, fast-adapting
Merkel’s discs
fine touch - in dermis, small receptive fields, slow-adapting
Ruffini’s ending
stretch - in the hypodermis, large receptive fields, slow adapting
Pacinian corpuscle
vibration/pressure - hypodermis, large receptive fields, fast-adapting
where are receptor cell bodies located?
dorsal root ganglion
what are skin receptor potentials?
graded potentials at the input layer (opens Na channels through stretch receptors) → APs at cell body
TRPV1
C fibres - binds to spicy foods, capsaicin
present in itch fibres
TRPM3
A delta fibres - when things get dangerously hot
CMR1
C fibres - binds to menthol
psychogeneic pain management
placebo - can have some ethical concerns
hypnosis - unaffected by opiate antagonists
pharmacological pain management
opiates - block opioid receptors in spinal cord, can have severe side effects
anti-inflammatory drugs - block prostaglandin at site of injury, side effects
cannabinoids - act in spinal cord/nociceptor endings, illegal in some places
stimulation pain management
acupuncture - sometimes affected by opiate antagonists
TENS - electrical stimulation activating endogenous opiates and blocking pain signals in spinal cord, inhibited by opiate antagonists
surgical pain management
cut peripheral nerve cord/cut dorsal cord/cord hemisection - create physical break in pain pathway, considerable risk of failure
frontal lobotomy - irreversible and severely affects behaviour
NSAIDS
non-steroidal anti-inflammatory drugs
act on COX pathway to reduce production of prostaglandins (reduces pain signals)
what does adaptation mean for the visual system?
we constantly are focusing on the main thing in our environment and not aware of most things in peripheral
interested in relative values, not absolute
eye to retina path
cornea → lens → virtuous humour → capillaries → retina → axons → photoreceptors
shows how we have evolved from our aquatic ancestors (as their paths are less messy)
photoreceptors to optic nerve
photoreceptors → bipolar cells → bipolar cells → amacrine cells → ganglion cells → optic nerve
how many rods in the retina?
~120 million
how many cones in the retina?
~ 7 million (most in the fovea)
S-cones
highest absorbance = 420 nm
rod wavelength sensitivity
450 nm
M-cones
highest absorbance = 530 nm
L-cones
highest absorbance = 560 nm
how do dogs see the world?
through dichromatic colour vision - saturation of yellow or bluish-gray
protanopia
loss of long wavelength cones - can’t see red
deuteranopia
loss of medium wavelength cones - can’t see green or red
tritanopia
loss of short wavelength cones - can only see redish and bluish colours
achromatopsia
loss of complete colour vision - usually a CNS issue
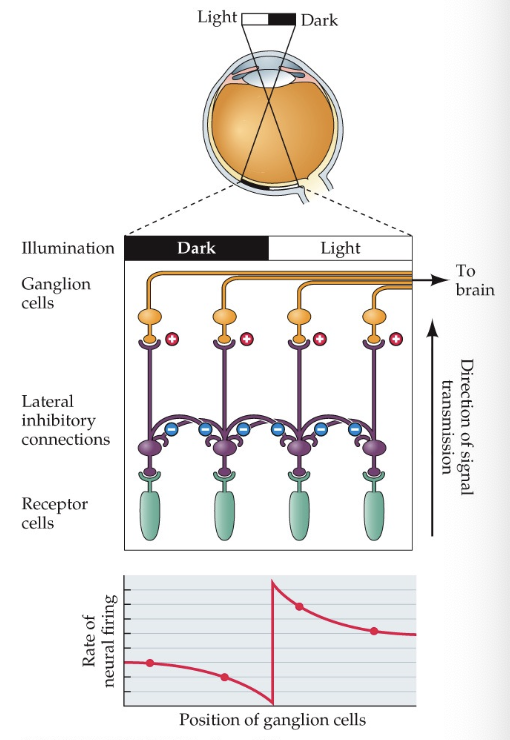
lateral inhibition
helps us detect contrast variation (Mach Bands) by inhibiting neighbouring photoreceptors and enhancing the photoreceptor that is being hit by light
only interest in relative values that contribute to context
enhances contrast discrimination
active process
receptive fields: inhibits light at peripheral in ON-centre cells to specify where the light is hitting
can mutually inhibit each other because of horizontal cells (opponent processes)
what happens to vision if the left optic nerve is cut?
no visual info is coming through the left eye
what happens if left optic tract is cut?
no info from the right visual field is coming in
what happens if the optic chiasm is cut down the middle?
we lose input from the nasal halves of the retina - lose peripheral vision
what are p-cells/parvocellular layers for?
perception - cones from fovea
what are m-cells/magnocellular layers for?
motion - rods and cones from periphery
what would happen to ocular dominance columns if an eye was blocked at young age?
dominance columns for the covered eye would shrink and grow for eye that is not covered
colour constancy
despite the background colour changing, all objects will remain the same colour to us

Young-Helmoltz trichromatic theory
Every colour we can see is made of a combo of red, green, and blue light - all of them together = white light
eye contains 3 types of cone cells
support: colour matching demonstrates that any colour can be matched by mixing red, green, blue
Hering’s opponent process theory
colour vision is based on three opposing colours
Red vs. Green
Blue vs. Yellow
Black vs. White
Vision can only perceive one colour at a time from each pair
afterimages: staring at a bright red object and then looking at a white surface = green afterimage
are trichromatic theory and opponent process theory mutually exclusive or complementary?
they complement each other
trichromatic: explains how cones detect light
opponent-process: explains how brain processes colour
damage to the ventral visual stream
difficulty recognizing what/who things are (agnosia)
can match bodily movements, but have difficulty recognizing what to do in a task
some cannot recognize familiar faces (Halle Berry neuron would not work)
damage to the dorsal visual stream
how difficulty with spatial orientation and motor tasks
can’t sort objects properly
difficulty with depth perception
non-conscious vision
still have circadian rhythm
still can be guided by light to do some photoreceptors attached to RGCs
can still guess what stimuli is and navigate a room with obstacles
driven by superior colliculus?