A1.1 - Water
1/18
Earn XP
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
19 Terms
Explain that water is the substance in which cells first developed and life’s processes still occur.
Water is essential for life because it provided the environment where cells first developed and continues to support life’s processes. It enabled the interaction and organization of molecules needed for life and the formation of compartments like membranes. Within cells, water acts as a solvent, allowing metabolic reactions to occur and maintaining the structure and function of the plasma membrane, ensuring life’s processes can continue. Water is vital for all living organisms as it creates an environment for cellular development and ongoing biochemical processes, facilitating molecular interactions and maintaining cellular structure.
Explain how a difference in electronegativity between two atoms results in a polar covalent bond.
A difference in electronegativity between two atoms causes a polar covalent bond because the more electronegative atom attracts the shared electrons more strongly. This unequal sharing creates a partial negative charge (δ⁻) on the more electronegative atom and a partial positive charge (δ⁺) on the less electronegative atom. In water, for example, oxygen is more electronegative than hydrogen, so the shared electrons are pulled closer to the oxygen atom, resulting in a polar molecule.
Draw two or more water molecules and hydrogen bonds between them with a notation to indicate polarity.
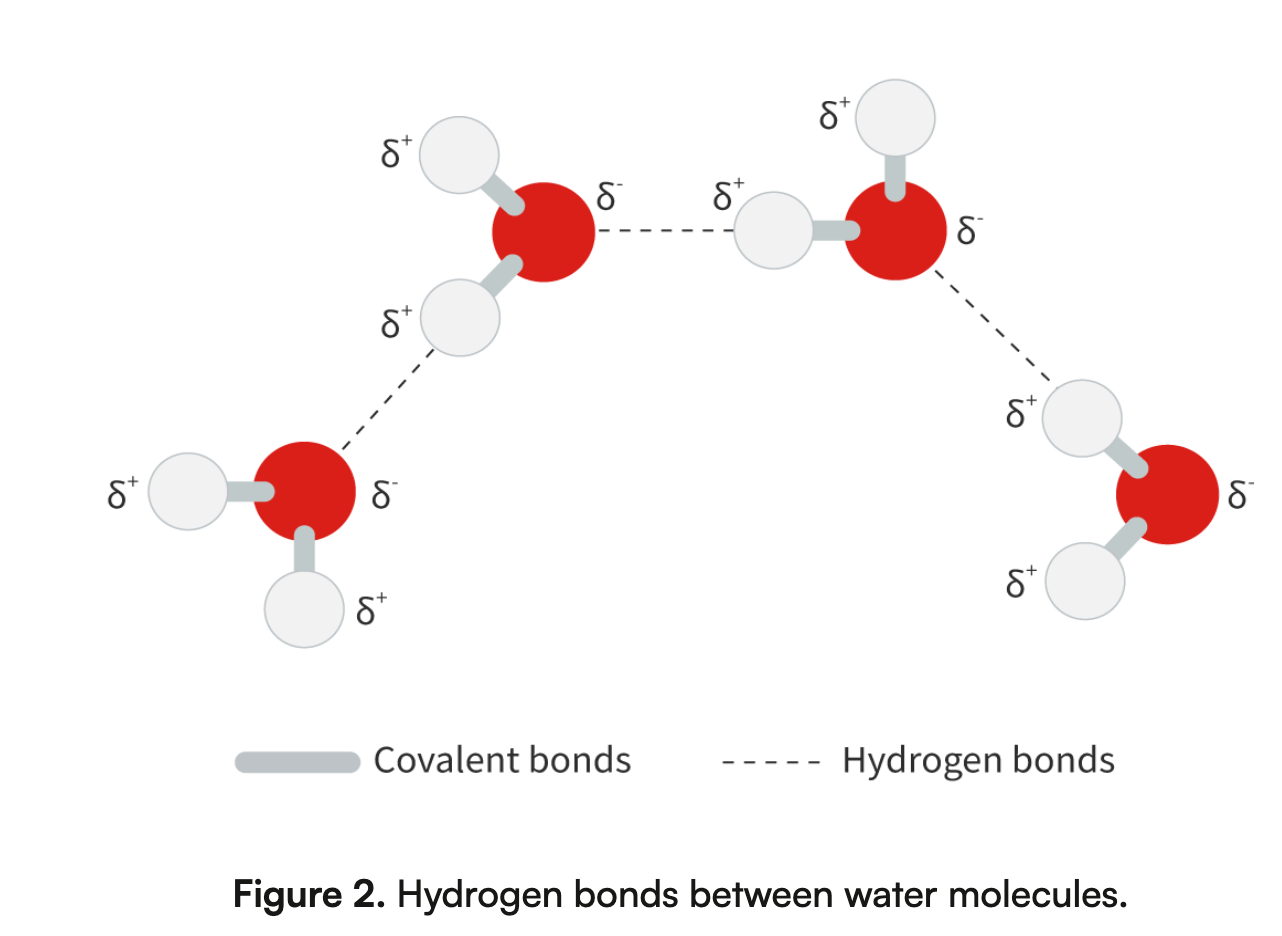
Explain that water molecules are attracted to each other and that this property, cohesion has important impacts on organisms.
Water molecules are attracted to each other through hydrogen bonds, where the partial positive charge (δ⁺) of hydrogen in one molecule is attracted to the partial negative charge (δ⁻) of oxygen in another. This property, called cohesion, allows water to resist external forces and stick together. Cohesion is crucial for organisms, as it enables processes like water transport in plants, where water is pulled up through the xylem against gravity, and supports surface tension, which allows certain insects to live on water surfaces.
Explain how the properties of water affect its roles as a means of transport.
Water's properties make it an effective means of transport essential for life. Its polarity allows it to dissolve many substances, such as salts, sugars, and amino acids, enabling these materials to be transported in aqueous solutions like blood plasma or plant sap. Water's cohesion and adhesion support capillary action, which helps move water through narrow spaces, such as plant xylem and soil pores, ensuring that nutrients and moisture reach cells. Additionally, water's ability to carry dissolved materials is critical for transporting essential molecules within organisms, such as glucose in blood or sucrose in phloem sap.
Explain how the solvent properties of water are linked to its role as a medium for metabolism.
Water’s solvent properties are essential for its role as a medium for metabolism because they allow many molecules to dissolve and interact within cells. Being polar, water can dissolve hydrophilic substances such as salts, sugars, and amino acids, which are the substrates for metabolic reactions. In the aqueous cytoplasm, dissolved enzymes catalyze these reactions, enabling processes like energy transfer, molecule synthesis, and waste removal. Additionally, water helps maintain the structure and function of enzymes, such as amylase, which require a hydrated environment to work effectively. This makes water indispensable for sustaining the chemical processes that support life.
Explain how the cohesive and adhesive properties of water are linked to its role as a medium for transport in plants and animals.
The cohesive and adhesive properties of water are essential for its role as a medium for transport in plants and animals. Cohesion, the attraction between water molecules due to hydrogen bonding, allows water to form continuous columns, such as in plant xylem, enabling it to move upward against gravity. Adhesion, the attraction between water and other surfaces, helps water cling to the walls of xylem vessels and soil particles, facilitating capillary action. In animals, cohesion and adhesion contribute to the efficient movement of water-based fluids like blood and lymph, ensuring the transport of nutrients, oxygen, and waste throughout the body.
Describe the properties of water including buoyancy, viscosity, thermal conductivity and specific heat capacity.
Properties of Water:
Buoyancy: Water's high density provides strong buoyant force, counteracting gravity and allowing less dense materials, like seal blubber, to float. This property supports aquatic organisms and aids movement in water.
Viscosity: Water has a low viscosity compared to other liquids, making it easier for organisms like seals and loons to move through it efficiently. However, it is more viscous than air, requiring streamlined shapes to reduce drag.
Thermal Conductivity: Water has a high thermal conductivity, which allows heat to transfer quickly. This can lead to heat loss in organisms like seals, which rely on insulating blubber to retain body heat while in water.
Specific Heat Capacity: Water has a high specific heat capacity due to hydrogen bonding. This means it requires significant energy to change temperature, helping stabilize aquatic environments. In contrast, air has a much lower specific heat capacity, allowing insulated environments like seal lairs to retain heat more effectively
Contrast, using examples, how the physical properties of water and air have consequences for animals that live in these two habitats.
The physical properties of water and air present distinct challenges and advantages for animals living in each environment. Here’s a contrast of how these properties affect animals:
1. Buoyancy:
Water: Water’s high density provides buoyancy, which helps aquatic animals like ringed seals to stay afloat. Seals have low-density blubber that increases their buoyancy, enabling them to swim efficiently in the water.
Air: Air, being much less dense than water, has much lower buoyancy. This reduces the support available to animals on land or in the air. For example, a seal would find it more difficult to move efficiently on solid ice compared to swimming in water because the air offers no buoyancy.
2. Viscosity:
Water: Water’s relatively high viscosity compared to air creates drag, making movement slower. For animals like ringed seals, which have streamlined bodies, the viscosity of water offers resistance, but their shape helps reduce drag, allowing for efficient swimming.
Air: Air has a much lower viscosity, which allows for easier movement for flying animals like birds. The black-throated loon, for example, has an aerodynamic body that allows it to glide smoothly through the air with minimal resistance. However, the low viscosity of air does not aid buoyancy, requiring the loon to use other adaptations like feathers for lift.
3. Thermal Conductivity:
Water: Water has high thermal conductivity, which can lead to rapid heat loss for animals like seals that live in cold waters. To combat this, seals have insulating blubber to reduce heat loss.
Air: Air has much lower thermal conductivity than water, meaning it doesn’t transfer heat as efficiently. Animals like the black-throated loon use air for insulation by trapping it with their down feathers, preventing heat loss in colder climates.
4. Specific Heat Capacity:
Water: Water’s high specific heat capacity means it takes a large amount of energy to change its temperature. This property helps maintain stable temperatures in aquatic environments, providing a more consistent habitat for organisms like seals. However, it can lead to heat loss for animals in the water, as the surrounding water absorbs their body heat.
Air: Air has a much lower specific heat capacity, meaning it changes temperature more easily. This can create more extreme temperature fluctuations for animals living in the air or on land. The black-throated loon, for example, relies on feathers to insulate its body and regulate temperature, as the air can rapidly shift between hot and cold.
Summary:
In summary, the physical properties of water and air create both challenges and adaptations for animals living in each habitat. Water's buoyancy and viscosity support aquatic life but also lead to heat loss, while air’s lower density and viscosity aid movement for flying and land-dwelling animals but offer less thermal insulation. Adaptations, such as blubber in seals and feathers in loons, help these animals thrive despite the different physical properties of water and air.
Outline that the origin of water on Earth is understood to be extraplanetary, delivered by multiple collisions of water-rich asteroids.
The origin of water on Earth is believed to be extraplanetary, meaning it likely came from space. Scientists suggest that water was delivered to Earth through collisions with water-rich asteroids, also known as comets, during the early formation of the planet. These collisions occurred as Earth was cooling, and the resulting water condensed on the surface. The constant impact of smaller objects, such as meteoroids, also contributed to the accumulation of water over time. This extraterrestrial delivery of water played a crucial role in creating the conditions necessary for life to evolve on Earth.
Describe how the moderate temperature and gravity of Earth allow water to be retained.
Earth's moderate temperature and gravity are key factors in allowing water to be retained on the planet’s surface. As Earth cooled after its formation, liquid water was able to condense, and the planet's gravity helped keep it from escaping into space. Earth's gravity is strong enough to hold water in place, preventing it from being lost to the vacuum of space, which could happen on smaller planets or moons with weaker gravity. Additionally, Earth's distance from the Sun places it in the Goldilocks zone, where temperatures are not too hot to cause water to boil away or too cold to freeze it, maintaining liquid water in stable conditions.
Explain the concept of the ‘Goldilocks zone’ in relation to the search for extraterrestrial life.
The Goldilocks zone, also known as the habitable zone, refers to the region around a star where conditions are just right for liquid water to exist on a planet's surface—not too hot and not too cold. This concept is crucial in the search for extraterrestrial life because liquid water is considered essential for life as we know it. If a planet or moon is within the Goldilocks zone of its star, it has the potential to support life by maintaining stable, habitable temperatures that allow water to remain in its liquid form. For instance, Earth is in the Sun's Goldilocks zone, which is why it supports life. Scientists focus on identifying other planets in similar zones around stars in other solar systems, hoping to find extraterrestrial environments where life could exist.
_______ is the sum of a cell’s chemical reactions, which must take place in liquid water.
Metabolism
A water molecule is held together by ______ bonds, while different water molecules are temporarily connected by ______ bonds.
polar covalent, hydrogen
Water’s attraction to itself is called ______, while water’s attraction to other polar or charged surfaces is called _____.
cohesion, adhesion
Polar and charged substances are called ____. or ‘water-loving’, while non-polar substances are called _____.
hydrophilic, hydrophobic
Water counteracts the force of gravity on submerged objects due to ______, while the energy necessary for water to change its shape is its ______.
buoyancy, viscosity
Water helps moderate temperature because it has a high _______, while it also has a high ________ which makes it hard for artic animals to stay warm.
specific heat capacity, thermal conductivity
Most of the water on Earth is believed to have been brought by _______. Liquid water occurs on ______ that are in the _______ around a star
asteroids, planets, goldilocks zone