Chemistry (UNFINISHED!!!)
1/63
Earn XP
Description and Tags
all modules
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
64 Terms
Homogenous Mixture
Uniform mixture composition throughout; not composed of different substances (e.g air)
Heterogenous Mixture
Not a uniform mixture composition throughout; composed of different substances (e.g concrete)
What are the physical properties?
Size
Boiling Point
Condensation Point
Water Solubility
Magnetisim
Melting Point
Electrical Conductivity
Density
Immiscibility
Inorganic Chemicals
Not carbon derived. Includes all salts (e.g sodium chloride, magnesium bromide, carbon dioxide, sodium carbonate)
Organic Chemicals
Carbon derived (e.g. alkanes, alkynes, alcohols, aldehydes, ketones)
Ionic Compound
Cation+ and Anion-
Binary Compounds:
Cation named first, anion ends in ‘-ide’
(E.g Sodium Chloride)
Covalent Compounds
Between nonmetals.
1. More electronegative element last
Last element suffix ‘ide’
Prefix denoting the number of atoms
Covalent Compound Prefixes
Mono
Di
Tri
Tetra
Penta
Hexa
Hepta
Octa
Nona
Deca
Basic Radicals
Bicarbonate HCO3-
Hydroxide OH-
Nitrite NO2-
Nitrate NO3-
Sulphate SO4-2
Carbonate CO3-2
Phosphate PO4-3
Ammonium NH4+
Cation
Positively charged species; atom lost one or more electrons.
Anion
Negatively charged species; atom gained one or more electrons.
Electronegativity
Tendency to attract bonding electrons.
Group Classifications
Group 1: Highly reactive, low mpt/bpt, low density
Group 2: Shiny, silvery in colour, low density, somewhat reactive
Group 18: Colourless, odourless, inert and singular
Ionic Network
A network (lattice/crystal) of positive and negative ions bonded by strong electrostatic forces between two oppositely charged ions
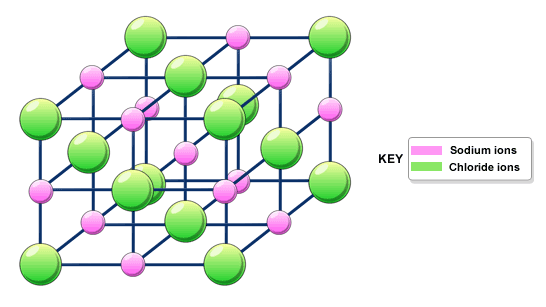
Ionic Network Properties
Hard (electrostatic force attraction)
Brittle/non-malleable (force causes fracturing due to ion repulsion)
High mpt/bpt (strong ionic bonds)
Metallics
‘Sea’ of delocalised electrons surrounding positive nuclei of metal atoms; nucleus close together.
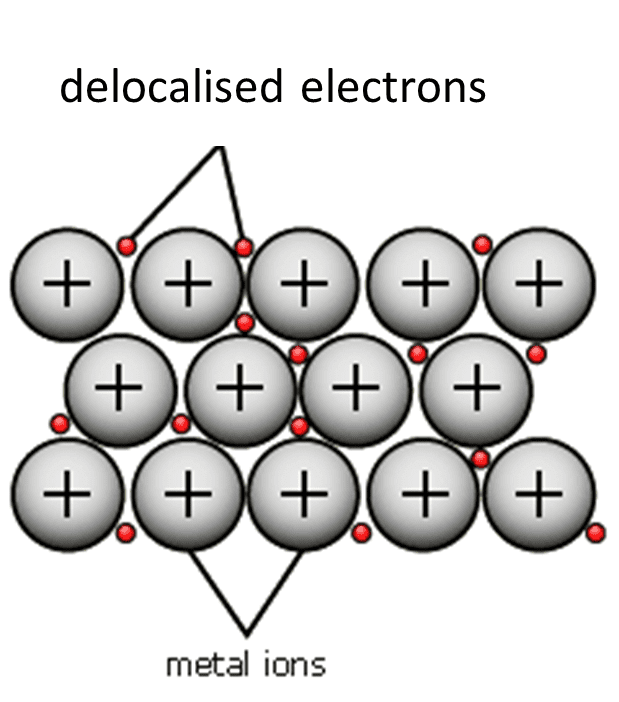
Metallics Properties
Malleable (electron movement prevents fracturing)
Conduct electricity (electron flow)
Strong (electrostatic forces)
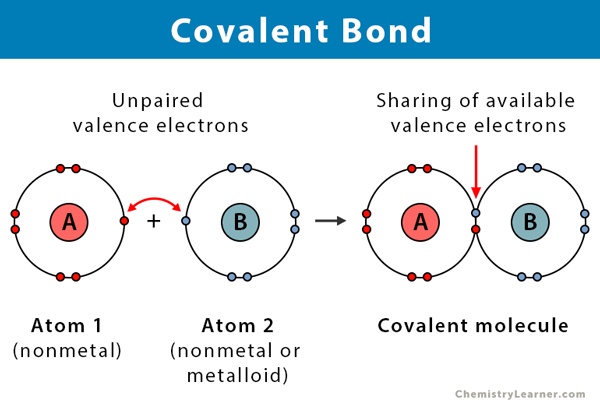
Covalent Bonds
Involves sharing of electrons between two or more non-metals to create a noble structure (e.g water)
Strong intramolecular forces holding atoms together
Weak intermolecular forces (between neighbouring molecules), resulting in low mpt/bpt
Covalent Networks
Atoms bonded in a 3D network structure.
High mpt/bpt (e.g diamond, graphite, silicon dioxide)
Percentage Composition Formula

The Mole
A very big number (6.022 x 1023) called Avagadro's number.

Acid + Base
= Salt + Water
E.g Hydrochloric Acid + Sodium Hydroxide → Sodium Chloride + Water
HCl(aq) + NaOH(aq) → NaCl(aq) + H2O(l)
Acid + Metal Carbonate
= Salt + Water + Carbon Dioxide
E.g Hydrochloric Acid + Copper (II) Carbonate → Copper (II) Chloride + Water + Carbon Dioxide
2HCl(aq) + CuCO3(s) → CuCl2(aq) + H2O(l) + CO2(g)
Acid + Metal
= A salt + Hydrogen
E.g Hydrochloric Acid + Magnesium → Magnesium Chloride + Hydrogen
2HCl(aq) + Mg(s) → MgCl2(aq) + H2(g)
Decomposition
Metal Carbonate –(heat)--> Metal Oxide + Carbon Dioxide
E.g Copper (ii) Carbonate → Copper (ii) Oxide + Carbon Dioxide
CuCO3(s) –(heat)-- CuO(s) + CO2(g)
Precipitation
= Solution 1 + Solution 2 → Solid ppt + Solution 3
E.g Lead (ii) Nitrate + Sodium iodide → Lead (ii) iodide + Sodium nitrate
Pb(NO3)2(aq) + 2NaI(aq) → PbI2(s + 2NaNO3(aq)
Metal + Oxygen
= Metal Oxide
E.g Magnesium + Oxygen = Magnesium Oxide
2Mg(s) + O2(g) → 2MgO(s)
Combustion - 'Complete'
Hydrocarbon + Oxygen (INXS) → Carbon Dioxide + Water
E.g Propane + Oxygen → Carbon Dioxide + Water
C3H8(g) + 5O2(g) → 3CO2(g) + 4H2O(l)
Incomplete Combustion
Hydrocarbon + Oxygen (LR) → Carbon Dioxide + Water + 'Various' (e.g Soot)
E.g Octane + Oxygen → Carbon + Carbon Dioxide + Water
C3H18(g) + 5/2O2(g) → 7C(s) + CO2(g) + 9H2O(l)
Displacement
Metal 1 + Salt-Solution 1 → Metal 2 + Solution 2
E.g Zinc + Copper (ii) Sulphate → Copper (ii) + Zinc Sulphate
Zn(s) + CuSO4(aq) → Cu(s) + ZnSO4(aq)
Molarity
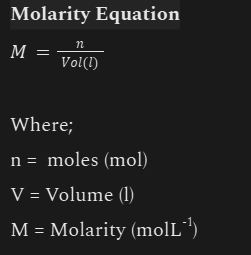
n(gas)
1 mol IDEAL gas
0℃ - 22.71 (STP)
25℃ - 24.79 (SLC)
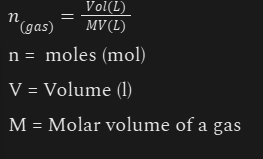
Dilution

Measures of Concentration - mol/L to g/L
mol/L * MM = g/L
mol/L * g/mol = g/L
Measures of Concentration - g/L to %w/v
g/L / 10 = g/100mL
g/100mL = %(w/v)
Measures of Concentration - mol/L to ppm
Multiply molarity by MM to get g/L
Multiply by 1,000 to convert g/L to mg/L (ppm)
Measures of Concentration - %(V/V)
Percentage volume represents the concentration of a solute in a solution where the solute and the solution are both liquids.
Boyle’s Law
At constant temperature, the volume of the gas increases as the pressure decreases. The volume of the gas decreases, pressure increases.
P1V1 = P2V2 || Where (P)ressure and (V)olume
BLT - Boyle's, Constant Temperature
Charles's Law
At constant pressure, volume of gas increases as temperature of gas increases and volume decreases when temperature decreases.
CP - Charles, Constant Pressure
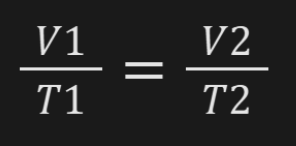
Gay-Lussac's Law
Fixed gas at constant volume, pressure increases linearly with temperature.
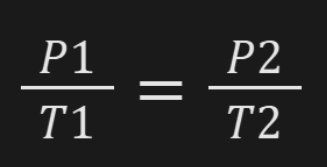
Combined Gas Law
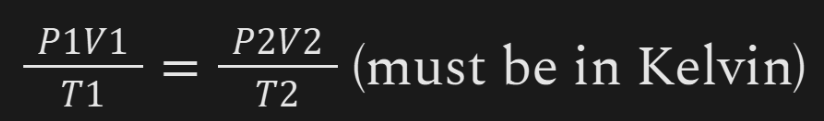
Avagadro's Law
Equal volume of gases at same temperature and pressure contain equal numbers of molecules.
n(gas) = Vol(L) / MV(L)
Ideal Gas
Ideal gas contains a large number of molecules which travel in a random, rapid motion - moves in straight lines until hitting a wall.
A gas is only ideal if its molecules occupy a negligible amount of the container.
Ideal Gas Law
PV = nRT
P = Pressure | V = Volume | n = mol | R = 8.314K-mol-1 | T = Temperature
(Boyles + Charles + Avagadro)
Room Temperature State of Matter
Unlisted: Solid
Bromide and Mercury: Liquid
Oxygen, Nitrogen, Fluoride and Chlorine + Group 18: Gas
Chemical Reactions
Physical Change | Chemical Change | |
New substances formed? | No | Yes |
Bonds broken | No (intermolecular attraction weakens) | Yes (intramolecular bonds broken) |
Energy needed | Relatively small | Relatively large |
E.g boiling versus electrolysis of water | 2 single covalent bonds - intramolecular | Bonds broken |
Equation | H2O(l) –(heat)--> H2O(g) | 2H2O(l) –(electricity)--> 2H2(g) + O2(g) |
Advantages / Disadvantages of molymod kit?
Ad:
Visualised relative kinetic energy
Breaking of bonds/rearrangement
Double covalent bonds
Dis:
Can’t see energy
Electron movement
Not to scale
AATSI Detoxification
Outer Cycad Fruit
Edible through anaerobic fermentation (decomposition) - soaking + burying which eliminates macrozamin toxin (carcingoenic)
Inner Cycad Seed
Leaching process - cut open and washed with running water - removes cyasin carcinogen
Rate of Reaction
Rate which reactants are used up OR rate at which products are formed
Factors affecting Rate of Reaction
Temperature
Reactant surface area
Concentration of reactants
Catalysts
Exothermic Reactions
Release heat.
Intermolecular Bonding
Occurs between covalent molecules. Three types:
Dispersion (weakest)
Dipole-dipole (Between atoms of different electronegativities)
Hydrogen bonding (strongest, between H-O, H-N, H-F)
q = mCΔT
Q = Joules
M = Mass (g)
C = Specific heat capacity (4.18)
ΔT = Temperature Change
Metals in Water (Active)
React with H2O to form a metal hydroxide and H2.
Vigorous in any water - store in oil
Sodium + water → sodium hydroxide + hydrogen gas
Less Active Metals in Water
Group 2 and 3 metals react less explosively in water
Magnesium + Water → Magnesium Hydroxide + Hydrogen Gas
Even Lesser Active Metals in Water
Not with hot water but steam
Aluminium + Steam → Aluminium oxide and Hydrogen Gas
Metals and Acids
Most metals (unreactive silver/gold or chemically inert like platinum excluded) react with acids, including dilute HCl and H2SO4 to produce a salt + hydrogen. More reactive react more vigorously.
Half Equations
In many chemical equations, electrons are lost (oxidisation) and electrons are gained (reduction) between one species in the reaction and the other.
Oxidisation Half Equation
Mg(s) → Mg+2 + 2e-
Reduction Half Equation
2H+(aq) + 2e- → H2(g)
Galvanic Cells
Voltaic cells; convert chemical energy into electrical energy. Allows for external flow of electrons. Used for reactivity testing.
Zn(s) → Zn(aq)+2 + 2e- | OXIDATION half equation.
Cu+2 + 2e- → Cu(s) | REDUCTION half equation.
How do you determine the Theoretical Voltage of a Galvanic Cell?
Find electron ions on the reduction table.
Determine which ion will be the one that oxidises (closest to K+)
Flip equation and change the sign of its voltage.
Leave the reduction half equation and voltage as is.
Add each half equation and voltage to determine the EMF of the cell.
Oxidation States Rules
Elements are given an oxidation state of zero
The oxidation number of a simple ion is the same as its charge
Hydrogen = +1 when forming compounds with non-metals. Hydrogen = -1 in metal hydrides
For polyatomic ions the oxidation numbers must add up to the charge on the ion
Oxidation State Golden Rule
If an oxidation number increases, then oxidation is occurring.
If an oxidation number decreases, then reduction is occurring.