Animal Cell Biology Exam 1
1/63
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
64 Terms
basic concepts of proteins
phosphocarrier protein HPr,
aspartic acid
Asp, negative
glutamic acid
Glu, negative
arginine
Arg, positice
lysine
Lys, positive
histidine
His, positive
asparagine
Asn, uncharged polar
glutamine
Gln, uncharged polar
serine
Ser, uncharged polar
threonine
Thr, uncharged polar
tyrosine
Tyr, uncharged polar
alanine
Ala, nonpolar
glycine
Gly, nonpolar
valine
Val, nonpolar
leucine
Leu, nonpolar
isoleusine
Ile, nonpolar
proline
Pro, nonpolar
phenylalanine
Phe, nonpolar
methionine
Met, nonpolar
tyrptophan
Trp, nonpolar
Cysteine
Cys, nonpolar
what charges form the covalent bond
alpha carbon (get rid of O-) and N (get rid of 2 H+)
how is a peptide covalent bond formed
formed with the removal of water and is on a single plane by the resonance
what are the two ends of a protein chain
amino-terminis (N-terminus) and carboxy terminis (C-terminus)
what are the components of the polypeptide backbone
C, H, O and N
what are the components of the side chains (amino acid residues)
the R group
what are the four classes of amino acid side chains
non-polar (hydrophobic), polar, acidic, basic
Ala side chain (nonpolar)
CH3
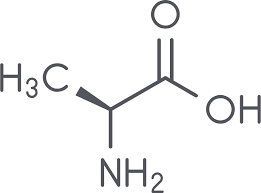
Val side chain (nonpolar)
CH3 - CH - CH3
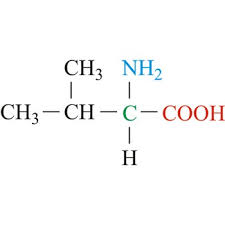
Leu side chain (nonpolar)
CH2 - CH - (CH3)2
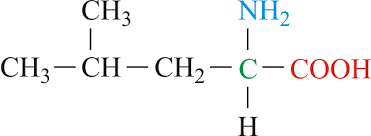
Ile side chain (nonpolar)
CH3 - CH - CH2 - CH3
Pro side chain (nonpolar)
look on slide 5
phe side chain (nonpolar)
CH2 - ring
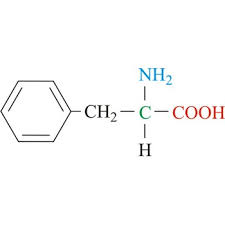
met side chain (nonpolar)
CH2 - CH2 - S - CH3
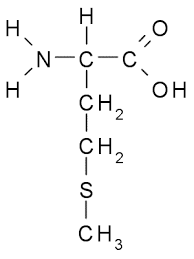
trp side chain (nonpolar)
CH2 - 2 rings
gly side chain (nonpolar)
H
cys side chain (nonpolar)
CH2 - SH

asn side chain (polar)
CH2 - C - O and NH2
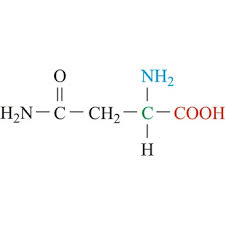
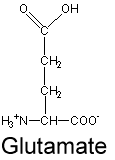
glu side chain (acid)
CH2 - CH2 - C - O and O-
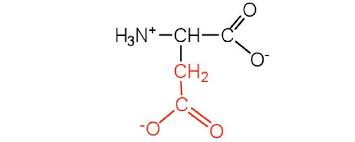
asp side chain (acid)
CH2 - C - O and O-
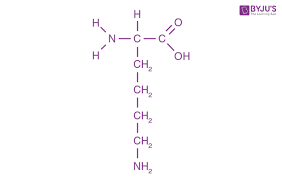
lys side chain (basic)
CH2 - CH2 - CH2 - CH2 - NH3+
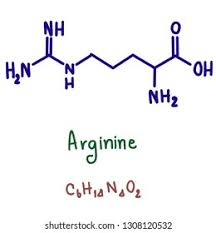
arg side chain (basic)
CH2 - CH2 - CH2 - NH - C - NH2+ and NH2
his side chain (basic)
CH2 - ring
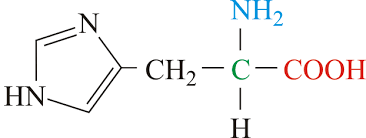
ser side chain (polar)
CH2 - OH
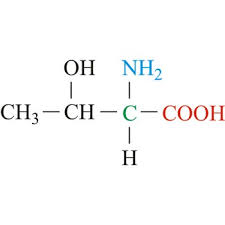
thr side chain (polar)
CH3 - CH - OH
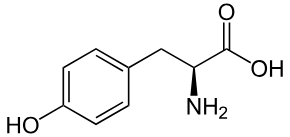
tyr side chain (polar)
CH2 - ring - OH
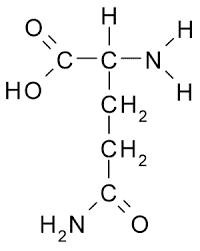
gln side chain (polar)
CH2 - CH2 - C - O and NH2
4 weak noncovalent interactions
ionic bond, hydrogen bond, van der waals interaction, hydrophobic interaction
covalent interaction
disulfide (S-S) bond
hydrophobic interaction
polar charged (H2O) molecules attract each other in water; nonpolar molecules are excluded from water and are more stable when they come together