bis 102 protein stuff
1/33
Earn XP
Description and Tags
after mt1
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
34 Terms
separation of protein chains with:
extreme pH
8M urea
6M HCl
high salt concentration (like ammonium sulfate)
mer-capto-ethanol (MCE)
HS-CH2-CH2OH
protonates one cysteine and the other stays attached to MCE
sulfhydryl reducing agent
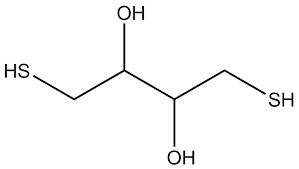
di-thio-threital (DTT)
C4H10O2S2
sulfhydryl reducing agent
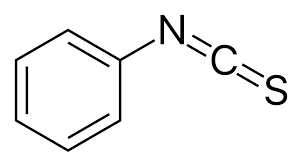
phenyl-iso-thio-cyanate (PITC)
N-terminal analysis
Edman’s reagent
produces phenyl-thio-hyantoins (PTH-aa)
carboxypeptidase A
C-terminal analysis (exo-peptidase)
cleaves any residue EXCEPT Pro, Arg, Lys
carboxypeptidase B
C-terminal analysis (exo-peptidase)
ONLY works with Arg, Lys
trypsin
cleaves Arg or Lys (proteolytic enzyme)
chymotrypsin
cleaves Phe, Trp, or Tyr, Leu (proteolytic enzyme)
clostripain
cleaves Arg (proteolytic enzyme)
staphylococcal protease
cleaves Asp or Glu (proteolytic enzyme)
endopeptidase Lys-C
cleaves Lys (proteolytic enzyme)
cyanogen bromide
cleaves Met (proteolytic enzyme)
phosphorylation
modifies: S, T, Y
hormone receptors
regulatory enzymes
acetylation
modifies: K
histone
metabolic enzymes
methylation
modifies: K, R
histones
acylation
modifies: C
G-protein coupled receptors
prenylation
modifies: C
Ras p21
ADP-ribosylation
modifies: H, R
G proteins
eukaryotic elongation factors
adenylylation
modifies: Y
glutamine synthetase
peptide planar bond
six atoms of the peptide group in a plane; C-N partial double bond and can’t rotate; two degrees of freedom
rotation parameters
phi: 180 degrees
psi: 180 degrees
ramachandran plot
phi vs. psi
proline: most tightly constrained and can’t react (no free N)
glycine: least sterically hindered
alpha-helix
hydrogen bonds
phi: -60 degrees
psi: -45 to -50 degrees
residues: 1.5 amps (0.15 nm)
3.6 residues per long-axis turn
6 amps in diameter
rise per turn (pitch): 5.4 amps
beta-pleated sheet
rise per residue:
3.47 amps ANTIPARALLEL
3.25 amps PARALLEL
2 residues per long-axis turn
hydrophobic interactions on one side of sheet
radial strands
strong/rigid and have high percentage of beta-sheets
circumferential strands
flexible and have high percent of alpha-helices
hydrogen bonding between…
carbonyl oxygen and amide in i+3 position
beta-turn
a tight loop from an H-bond between carbonyl O and amide H three positions down
makes this stable
allows protein to reverse direction of the peptide chain
types 1 (more common) and 2
involves four residues without specific phi and psi