Nuclear and Electronic Structure, Atoms and the Periodic Table
1/74
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
75 Terms
Equation for speed of light
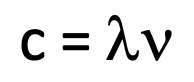
speed of light = wavelength x frequency
Energy of a photon
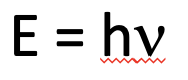
energy = planck’s constant x frequency of the light
Mass Defect
The atomic mass of every atom except hydrogen is less than the sum of the masses of protons. neutrons and electrons present.
A measure of the binding energy of the nucleus
How to calculate mass defect
the difference between the expected mass based on the number of protons, neutrons, and electrons and the observed mass of an atom
Binding energy
the energy released if all the protons, neutrons, and electrons were brought together
Equation to calculate energy equivalent to mass defect
E=mc2 (mass defect=m)
What is considered a large amount of energy?
Hydrogen bonds (weak) - 15 kJ/mol
C-C bond - 350 kJ/mol
Burning 1 mole of butane - 2.86x103 (large)
3 types of ionizing radiation
⍺, β, 𝛾
⍺ - radiation
A helium nucleus (atomic mass 4, atomic number 2 — 2 protons, 2 neutrons)
change in mass defect after alpha emissionn
β - radiation and emission
an electron
beta emission is the loss of an electron, mass number is almost unchanged, atomic number goes up by one - neutron changes to proton and gives out electron
Gamma radiation/emission
gamma radiation is electromagnetic radiation
it is emitted along with or after one of the other kinds of radiation when the new nucleus formed is in an excited state
gamma ray (hv)
excited/ high energy nucleus state is metastable, denoted by m
Nucleosynthesis
the process of making elements ( in the stars)
Big bang nucleosynthesis
hydrogen and helium were formed within the first few minutes of the start of the universe
big bang produced 1H, 2H (D), 3H, 3He, 4He and a small amount of 7Li.
the mechanism of the process is the fusion of protons and neutrons with themselves and with small nuclei
Stellar nucleosynthesis
heavier elements are made from hydrogen and helium in stars using the process of nuclear fusion —when two or more nuclei react to form one or more different atom
processes depend on temperature and mass of stars — older and larger stars can produce heavier elements up to iron with the triple alpha process and the alpha ladder where helium nuclei (alpha particles) fuse with eachother and with the heavier nuclei that they form
Supernova nucleosynthesis
A supernova creates a lot of energy, necessary to create elements heavier than iron
Interference (2 types)
Constructive: two waves in phase interfere and add to give a greater amplitude
Destructive: two waves out of phase interfere and cancel out to give reduced or zero amplitude
How to find energy of a mole of photons
multiply energy for one photon times avogadro’s number
The photoelectric effect
UV radiation is directed onto a metal surface and electrons will be ejected from the surface of the material if the frequency of the UV radiation is above a threshold value
Electron must have at least a certain energy (workfunction)
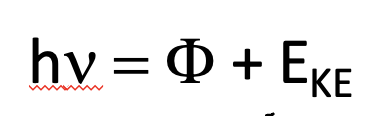
where EKE is the kinetic energy of the ehected photoelectron
Bohr’s quantized model equation
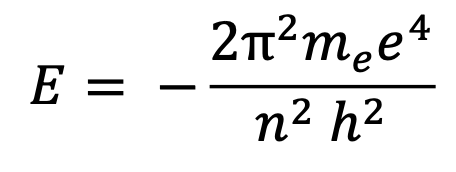
n is the principal quantum number— everything else is constant (me= mass of an electron; h= planck’s constant; e is the charge on the electron; pi)
The Bohr Atom
Electrons can only have particular quantized energies represented by the circular orbits
Quantization
Only certain energies are allowed, there are no “in between” energies (like a ladder)
possible energies labeled using letter n (principal quantum number)
Atomic spectrum of hydrogen
emission spectrum will have a line at hv = E2 - E1
The Rydberg equation
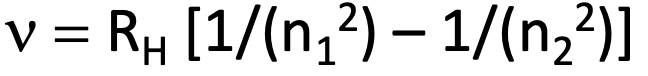
n is an integer used to number the energy levels
RH is the Rydberg constant •= 3.29 x 1015 Hz (but different on data card, in m-1
Main series to know
Lyman Series - n1 = 1 (emissions start in excited state and emit until at n=1)
Balmer Series - n1 = 2
Paschen Series - n1 = 3
Ionization energies
if n1 = 1 and n2 = infinity then you get the ionization frequency which can be converted to ionization energy
limitations of Bohr model
It is very simple and doesn’t fit atoms bigger than hydrogen very well
The de Broglie equation
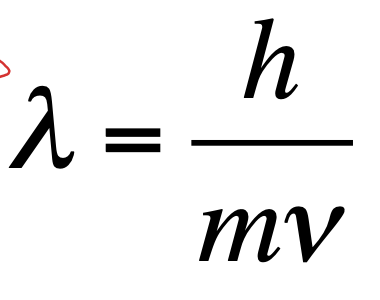
Waves can act like particles and particles can act like waves
•m = mass (kg) and v = velocity (ms-1)
•h = planck’s constant
Heisenberg Uncertainty Principle
Says that we can never accurately know the position AND momentum of an electron at the same time, so we cannot talk about the precise location of an electron, just the probability of finding it somewhere
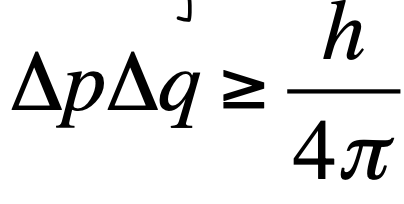
Where 𝚫p = uncertainty in momentum and 𝚫q = uncertainty in position
Wavefunction Ѱ
mathematical description of the wave to describe an electron
Ѱ2
The probability of finding an electron anywhere in a certain space
The Schrödinger Equation (time independent)
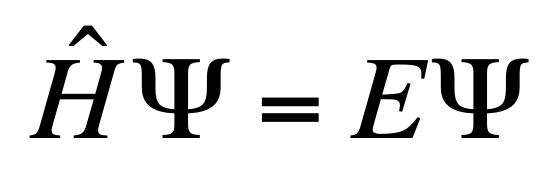
Where H is a Hamiltonian Operator (tells all conditions you need to know — shape, energy, direction of orbital — if you have all the conditions and Ѱ then you can find possible energies
Solution to the Schrödinger equation (only for hydrogen atom)
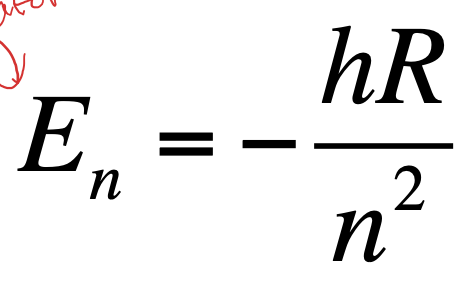
Where n is the principal quantum number and R is the Rydberg constant
It is the same as the Bohr equation but the R includes all the constants
Only possible to solve the equation exactly for two body systems
Four quantum numbers, what they tell us, allowed values
Overall: tell us about the energies and shapes of the electron wavefunctions called orbiatls
n: gives energy of orbital - can be any integer
l: gives shape of orbital - any integer 0 - (n-1)
l = 0 - s orbital
l = 1 p orbital
l = 2 d orbital
I = 3 f orbital
ml: orientation of orbital - any whole number from -l to +l
ms: spin (up/down, left/right ± 1/2)
Orbital
The region of space defined by Ѱ — the possible mathematical wavefunction
Orbital nomenclature
3px orbital (n = 3, l = 1,the x signifies orientation which is related to ml)
radial wavefunction R(r)
contains information about what happens to the wavefunction as the distance from the nucleus increases
Nodes
Where R(r) = 0
Amplitude of Ѱ changes sign
Radial distribution function
Related to R(r)2 — the probability of finding an electron in a certain location
The maximum value of the radia, distribution function is the most probable distance from the nucleus for the electron
Nodal planes in p orbitals
where the wavefunction is 0, where there is 0 probability of finding an electron
Nodal planes (and cones) in d orbitals
dxy, dyz, dxz all have nodal planes along axes (y and x; y and z; x and z)
dx2-y2 has nodal planes bisecting x and y axes
dz2 has two nodal cones instead of planes
Energy for a many electron atome
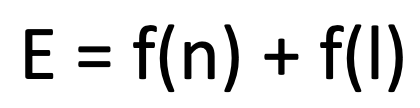
where f(n0 is a function that depends on the principal quantum number, n, and f(l) is a function that depends on the second quantum number, l
Pauli exclusion principle
Says that there cannot be any electrons that have the same set of 4 quantum numbers in the same atom because they will interfere with each other
Aufbau principle
The lowest energy orbitals are filled first (built up from bottom)
Hund’s rule
When filling a set of orbitals with the same energy (degenerate), electrons are added with parallel spins to different orbitals rather than pairing two electrons in one orbital
Pairing and exchange:
Pairing energy: the energy cost of putting two electrons in the same space (energy increases)
Exchange energy: a gain in energy when you can exchange identical electrons in degenerate orbitals (more exchanges is better, leads to lower energy: energy stabilization)
indistinguishable electrons occur where n, l, and ms are the same values
Stability of half and full shells:
half full and full are most stable: half full has most possible exchanges and no pairing energy; full has most possible energy minus max number of pairing energies. They maximize the benefit of exchange energy
Aufbau principle in transition metals
4s electrons slightly lower than 3d so they are filled before 3d, but when forming ions the 4s electrons are lost FIRST, before 3d
Limitation of Aufbau principle
If two orbitals are close in energy it can be energetically favorable not to obey aufbau principle but to obey Hunds rule
ex. Cr and Cu
Electron shielding
The energy of an electron mostly depends on how strong its attraction to the nucleus is; if another electron spends time between it and the nucleus then the first electron feels less of an attraction
When an electron wavefunction gets between the nucleus and another electron it can be said to be shielding. This is NOT interference, simply how much of the charge of the nucleus the electron is getting
Effective nuclear charge — definition and equation
How much of the charge of the nucleus an electron actually feels
Zeff = Z - S
where Z is the total nuclear charge and S is how much the electron is shielded by other electrons
Effective nuclear charge in 1s electrons
1s electrons do not feel the full nuclear charge as the two electrons shield each other — because electrons are wave not particles the shield each other even in the same orbital — also there is some shielding from 2s orbital because it has some electron density close to the nucleus
Intensity/level of shielding in different orbitals (penetration)
If an orbital has an intensity nearer to the nucleus than another orbital, then it is better at shielding
Penetration is the presence of a wavefunction’s intensity close to the nucleus
order of shielding strength by orbital
s>p>d>f
s electrons very good, f electrons horrible at shielding
effects of shielding in chemistry
d-block contraction: d orbitals are bad at shielding so as you go across the transition metals, each successive atom feels a larger Zeff so the size of the atoms shrink as you go across
types of bonding
Covalent - electron pair shared equally between atoms
Polar covalent- electron pair shared unequally between atoms
Ionic- electrons transferred from one atom to another
Three models of bonding
Lewis model
Valence Bond Theory
Molecular orbital theory
Lewis Model - the octet rule
Atoms in a molecule share electrons to complete a set of 8 electrons. Pairs of electrons not used in bonds are called lone pairs
Dative bond
type of bond where both shared electrons in a bond come from one atom
Limitations of the lewis model
Doesn’t work for some molecules at all (ex NO)
Doesn’t work for transition metal bonding
Doesn’t explain some properties (ex. why is O2 paramagnetic
Electronegativity
The power of an atom in a molecule to attract electrons to itself
atoms in the top right of the periodic table are the most electronegative
contributes to types of bonding (covalent= no difference in electronegativity; polar covalent= some difference; ionic= big difference)
Valence Bond theory — set of approximations it follows (from Schrödinger equation)
Orbitals (wavefunctions) can be combined on the same atom to form hybrid orbitals (if needed)
Orbitals can be combined to form (localized) bonds
Bonds can be the average of different resonance forms- useful to explain certain situations
Very good for explaining how chemistry happens, especially useful in organic chemistry
Molecular Orbital Theory
Atomic orbitals combine to form molecular orbitals that cna be delocalized over the whole molecule
Assumptions in MO theory
The orbital approximation — Linear combination of atomic orbitals (LCAO)
if two orbitals come close together they can interfere constructively or destructively
you cannot destroy or create/invent orbitals
In phase combination
Constructive interference
no nodal plane
bonding orbital
Out of phase combination
destructive interference ( subtraction represents an out of phase combination)
Has a nodal plane
antibonding orbital
Symmetry labels and interference
If orbitals have the same symmetry labels they can interfere, if they don’t, they cannot
Sigma orbitals vs pi orbitals
Sigma: σ Rotation about the nuclear axis does not change the shape of the orbital
Pi: rotation about the nuclear axis changes the shape of the orbital
Parity labels (g vs u orbital)
Even or odd symmetry:
g=gerade (even) — the phase of the orbital does not change upon inversion
u=ungerade(odd) — the phase of the orbital changes upon inversion (draw a line)
Energies of in phase vs out of phase orbitals
In phase (bonding) orbitals tend to have a lower energy
Out of phase (antibonding) orbitals tend to have a higher energy — antibody orbitals given a *
How to calculate bond order
#of filled bonding orbitals - #of filled antibonding orbitals
If bond order is positive when two atoms are combined:
then total energy must have gone down, ion/molecule can be created
non-bonding interactions
when bonding and antibonding interactions cancel out so there is no net interaction (ex. p orbital with sigma symmetry and p orbital with pi symmetry
LUMO
Lowest Unoccupied Molecular Orbital
HOMO
Highest Occupied Molecular orbital
when does paramagnetism occur
unpaired electrons
s-p mixing
when orbitals of the same symmetry can combine/interfere
destabilizes 3σg and 3σu* orbitals (pushes them much higher in energy)
Pushes 3σg orbital above 1piu* in energy
stabilizes 2σg and 2σu* orbitals (pushes them lower in energy)
*trick: goes from 3113 to 1313
s-p mixing stops (isn’t strong enough) after N2
Heteronuclear diatomics — differences
u and g symmetry labels do not apply
When orbitals have the same energy: MO has equal character of atom 1 and 2
When orbitals have slightly different energy: MO is nearer original energy of atom 2 (lower energy)
When orbitals have very different energy: MO is very near original energy of atoms 2 (lower energy)— has significant character of atom 2