iGCSE Organic Chemistry - DA
1/40
Earn XP
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
41 Terms
A chemical compound composed of carbon and hydrogen ONLY.
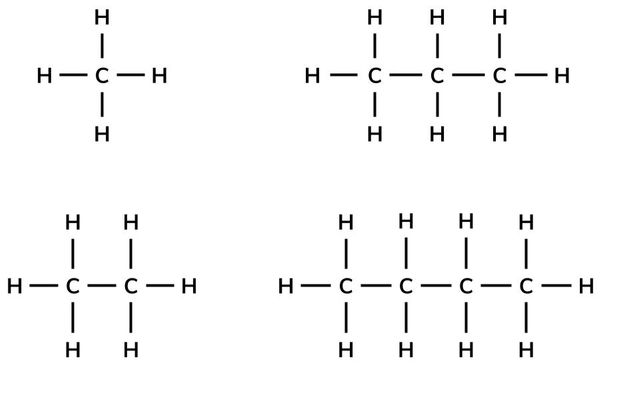
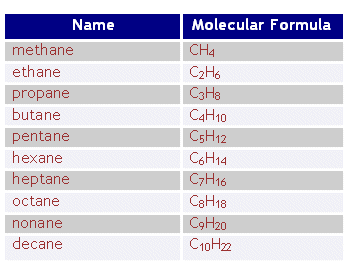
As the chain of a hydrocarbon gets longer, what happens to the viscosity?
It becomes more viscous
As the chain of a hydrocarbon gets longer, what happens to its flammability?
It becomes less flammable
As the chain of a hydrocarbon gets longer, what happens to its colour?
The colour gets darker (yellow to brown to black)
As the chain of a hydrocarbon gets longer, what happens to the intermolecular forces between the chains?
The intermolecular forces get stronger
As the chain of a hydrocarbon gets longer, what happens to its boiling point?
The boiling point increases as the chain gets longer
Why are alkenes useful?
They can be made into polymers
What is structural formula?
A structural formula displays the atoms of the molecule in the order they are bonded. e.g Hexane is CH3CH2CH2CH2CH2CH3