Gibbs Free Energy Study Guide
1/17
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
18 Terms
Metabolic pathway
A series of enzyme-catalyzed reactions in a cell that converts one substance into another, ultimately sustaining life
Catabolic vs anabolic pathways
Catabolic pathways: breaks down complex molecules into simple ones, releasing energy
• Anabolic pathways: builds complex molecules from simpler ones, requiring energy input
Define energy.
The ability to do work or cause change
What kind of energy is heat?
A form of energy transferred due to a temperature difference
What kind of energy is chemical energy?
Potential energy stored within the chemical bonds of molecules, like those in food, which is released or absorbed during chemical or absorbed during chemical reactions to power biological processes
First Law of Thermodynamics
The total energy in a system, like an organism, remains constant; energy cannot be created or destroyed, only transformed from one form to another
Second Law of Thermodynamics
In any energy transfer or transformation within a biological system or its surroundings, the total amount of entropy (disorder) always increases
What is entropy?
The measure of disorder or randomness within a biological system, or more broadly, the amount of energy that is unavailable to do work
What is a spontaneous process? How does entropy help to understand why certain processes occur without any input of energy?
A change in a physical or chemical system that occurs naturally and tends to happen without external energy input once initiated
Entropy explains this; systems naturally tend toward a state of higher entropy, or greater disorder
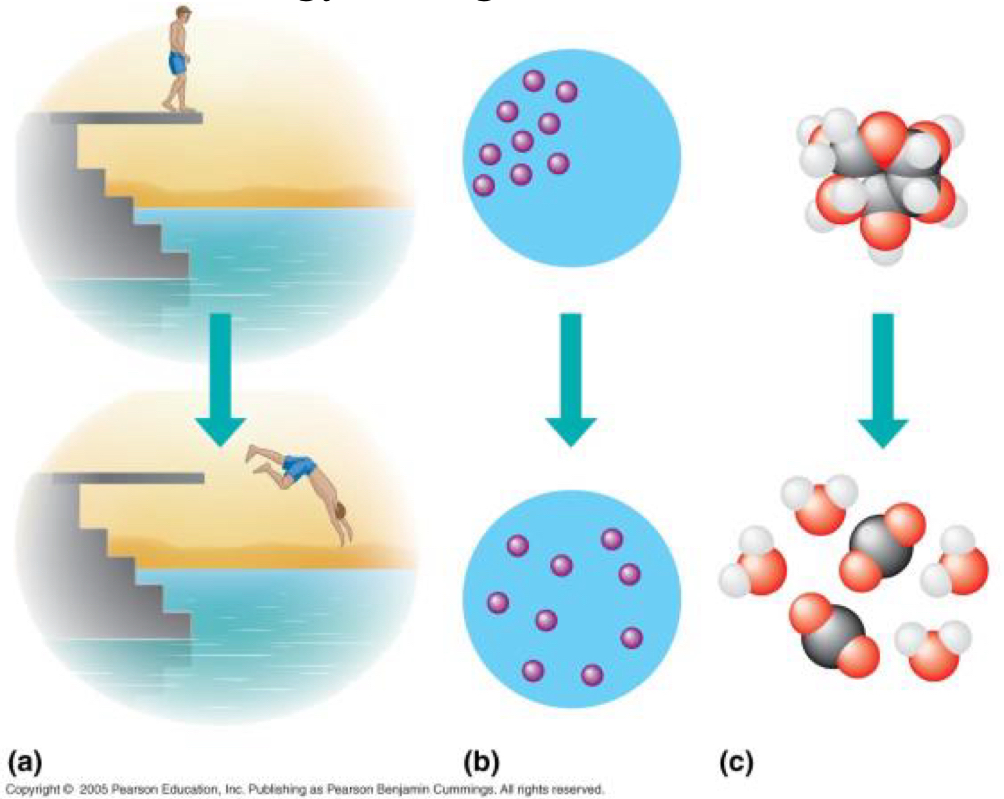
The free-energy change of a reaction tells us whether the reaction occurs spontaneously. Explain how each of the pictures in the top of each lettered diagram is an unstable system.
Solutes
• Explanation: The solute molecules (red dots) are highly concentrated in one area. This represents a system high concentration potential energy (a type of chemical potential energy).
• Instability: The system is unstable because the molecules will spontaneously spread out due to diffusion until they are evenly distributed. The disordered state (bottom) is more stable than the highly ordered, concentrated state (top) because it represents an increase in entropy (S), which is favored by spontaneous processes
Molecules
• Explanation: The top diagram shows two different types of molecules (the single red-and-white structure and the single red structure) that are separated and/or are in a high-energy, potentially reactive configuration.
• Instability: These molecules will spontaneously react to form a more stable compound (like the water molecules shown at the bottom). The products (bottom) have a lower bond energy or lower chemical potential energy compared to the reactants (top), indicating that the reaction releases energy and moves to a more stable state.
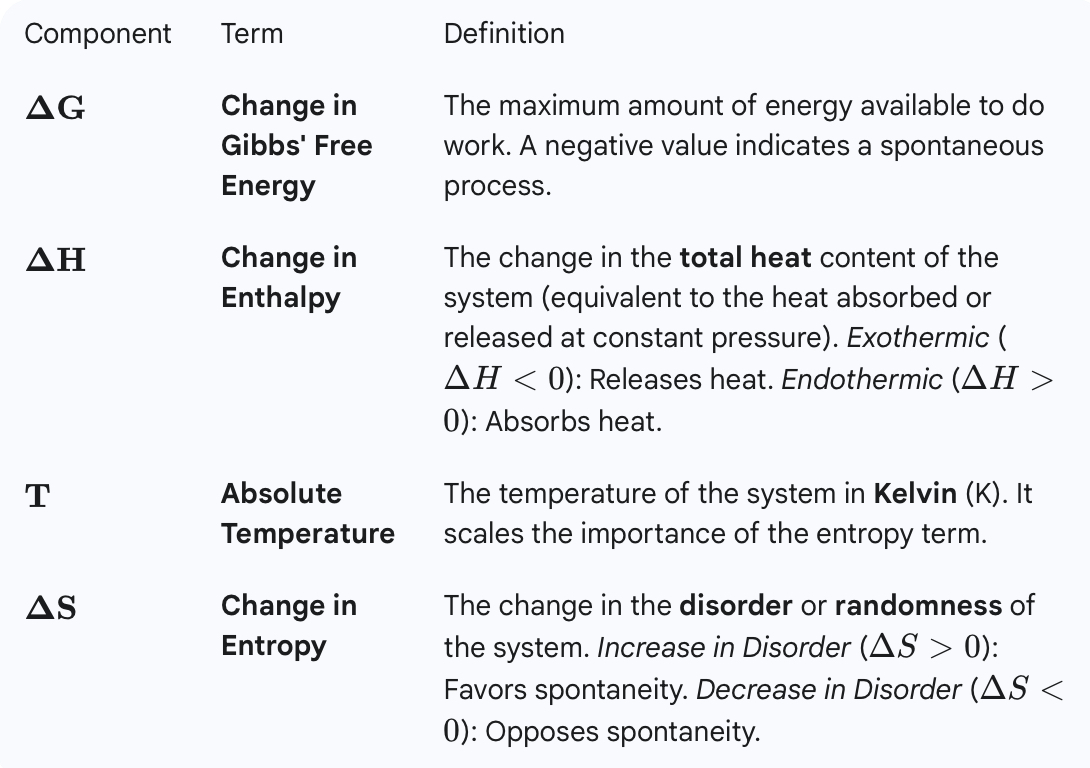
What is free energy? Write and define each component of the equation for free-energy change. (Equation: △G = △H - T△S)
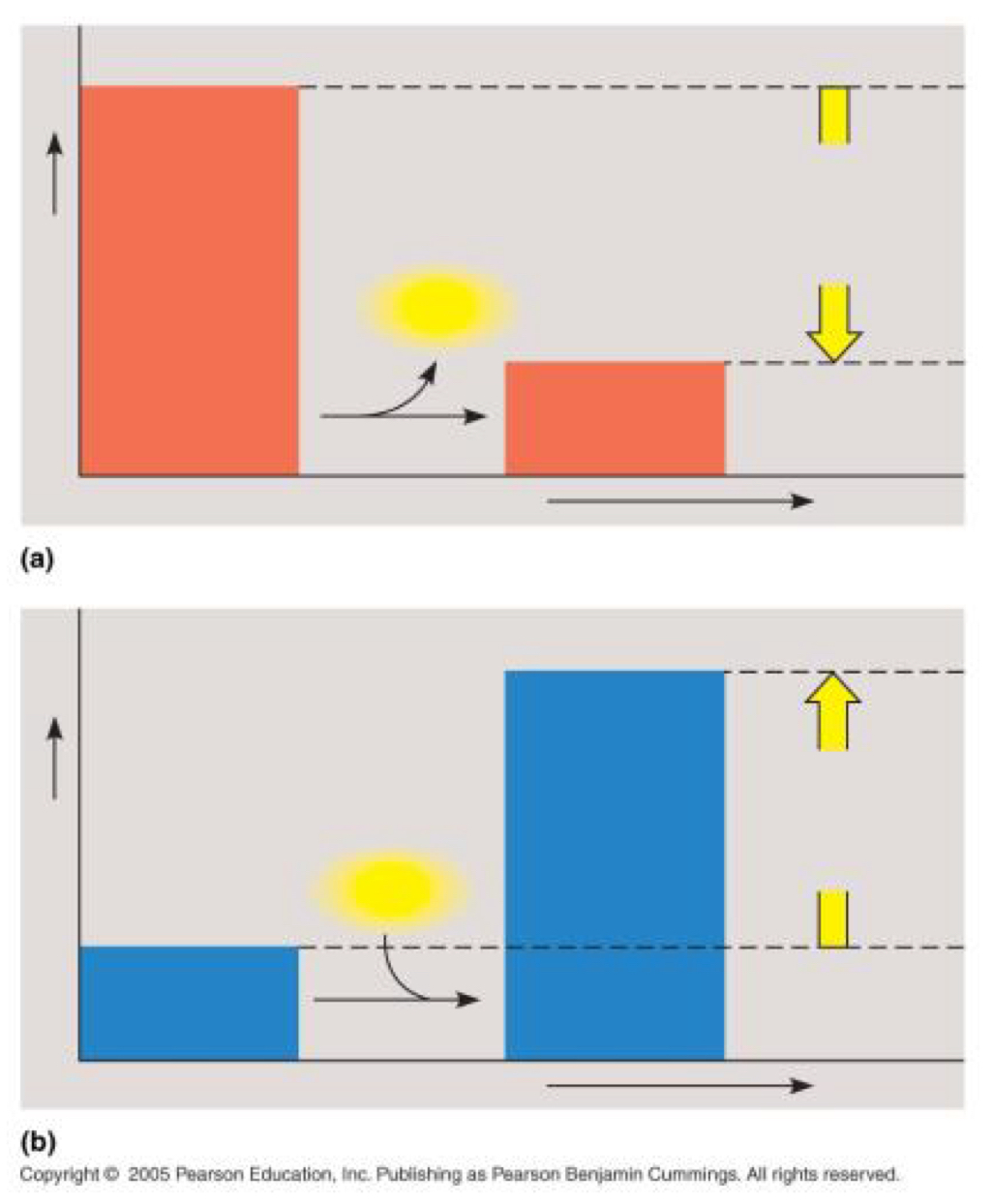
Which graph illustrates an endergonic reaction? Explain. Is △G negative or positive?
Diagram B - this graph shows that the products (right-side bar) have more Free Energy (G) than the reactants (left-side bar)
• In an endergonic reaction, energy is absorbed from the surroundings, meaning the system gains Free Energy.
• The change in Free Energy is calculated as: △G = G_products - G_reactants - since the change in the products are greater than the change in the reactants, the value of △G will be positive; these reactions are nonspontaneous.
Is cellular respiration an endergonic or an exergonic reaction? Explain. What is △G for this reaction?
Is an exergonic reaction
• In cellular respiration, glucose and oxygen are broken down to produce carbon dioxide, water, and ATP (energy). It is a catabolic process.
• The reaction releases energy (ATP, heat) into the surroundings, meaning the system loses Free Energy.
• Therefore, the change in Free Energy (△G<0). These reactions are spontaneous.
Is photosynthesis endergonic or exergonic? Explain. What is △ for this reaction? What is the energy source that drives it?
Is an endergonic reaction
• In photosynthesis, carbon dioxide and water are used to synthesize glucose (a higher-energy molecule) and oxygen. It is an anabolic process.
• The reaction requires an input of energy (it absorbs energy) to proceed, meaning the system gains Free Energy.
• Therefore, the change in Free Energy (△G>0).
• The energy source that drives photosynthesis is light energy (specifically, sunlight)
Explain the change in G for a drop of dye placed into a beaker of distilled water?
The process of a drop of dye dissolving and spreading throughout a beaker of distilled water is a spontaneous process, meaning the change in Free Energy (△G) will be negative (△G<0).
Is an example of diffusion, which is a spontaneous process driven by the tendency toward maximum entropy (disorder).
• Initial State: Dye is highly concentrated in one spot (low entropy); the system has a relatively higher G because a lot of work (energy) would be required to keep the molecules separated and ordered.
• Final State (Products): Dye molecules are evenly distributed throughout the water (higher entropy); the system has a relatively lower G because the molecules are more disordered and require less work to maintain this state.
“…A cell that has reached metabolic equilibrium is dead!” Explain what this statement means.
Means that a living cell must maintain a constant flow of energy and materials to survive; it must never reach equilibrium with its surroundings
Which one is an open system? Which one is a closed system?
A) Closed system; water is recirculating in a fixed, isolated container. Eventually, the water levels will equalize, and the turbine will stop spinning as the system reaches equilibrium
• C) Open system; the water is constantly flowing into the system from an external source (the ) and out of the system to the surrounds; water levels in the reservoirs will remain unequal, and the turbines will continue to spoil indefinitely as long as the flow is maintained, preventing the system from reaching equilibrium
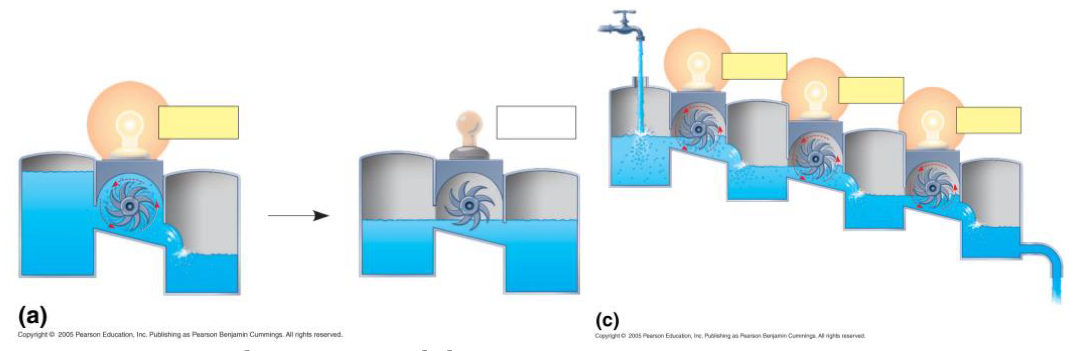
Which diagram represents the metabolism in a living system? Justify your answer.
Diagram C represents the metabolism in a living system
• Living organisms, including cells, are open systems that constantly exchange energy and matter with their surroundings
• Like the continuous flow of water in Diagram C, a cell maintains a constant inflow of reactants (like glucose) and an outflow of products (like CO_2 and water)
• This constant flow ensures that the cell's metabolic reactions are prevented from reaching equilibrium (like the equal water levels in Diagram a), allowing the release of Free Energy to drive essential life processes (represented by the constantly spinning turbines and lit light bulbs)