Module 6: Organic chemistry and analysis
1/110
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
111 Terms
aromatic compounds
compounds that contain benzene ring
benzene is
a major feedstock used in many industries: polymers, pharmaceuticals, dyes, explosives.
benzene itself is highly carcinogenic
simplest arene with an empirical formula of CH and a molecular formula of C6H6
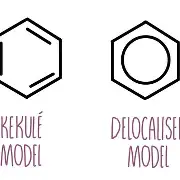
kekule model
a cyclic structure for benzene with 3 alternating carbon-carbon double bonds
disproving kekule model
The lack of reactivity of benzene
The length of the carbon-carbon bonds in benzene
Hydrogenation Enthalpy of benzene
The lack of reactivity of benzene
If benzene had C=C double bonds it would decolour bromine water in an electrophilic addition reaction.
But:
Benzene does not undergo electrophilic addition reactions
Benzene does not decolourise bromine under normal conditions, it requires a catalyst to react
This suggests there are no C=C double bonds in benzene.
bond length
can use x ray diffraction to determine bond length
prediction: C-C and C=C have different bond lengths so benzene would be an irregular hexagon if Kekulé’s model were true
BUT
benzene has a regular hexagon shape with equal bond lengths of 0.139nm which is in between the bond lengths of a C-C bond and a C-C bond
Hydrogenation enthalpy
the enthalpy change when one mole of an unsaturated compound reacts with an excess of hydrogen to become a fully saturated compound
Hydrogenation enthalpy of benzene
expected to have a enthalpy change of hydrogenation that is 3x the enthalpy change of hydrogenation of cyclohexene (-360kJmol-1)
- cyclohexene has 1 double bond and the Kekulé structure of benzene has 3 double bond
But:
The enthalpy change of hydrogenation of benzene is -208kJmol-1 therefore the actual structure of benzene is more stable than expected. Less exothermic than expected.
bonding if kekule was correct
3 of the carbon electrons in sigma bonds between each carbon-carbon and each carbon-hydrogen
remaining carbon electron in a p orbital adjacent to carbon atom resulting in alternating pi bonds between carbons where there is high electron density
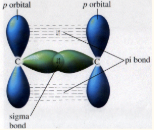
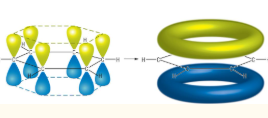
Delocalised Model of Benzene
Each carbon creates three covalent σ-bonds with 3 of its electrons.
The remaining electron is found in a p-orbital at a right angle above and below the carbon atom.
the p-orbitals overlap evenly creating a ring of delocalised π-electrons above and below the structure.
rings allow charge to be evenly spread across the molecule, making it stable and allowing equal bond lengths.
why doesnt benzene undergo electrophilic addition
Benzene has a lower electron density because the electrons are delocalised across the ring. This means it is less able to attract the electrophile and will be destabilised if groups are added to two neighbouring carbons
what reaction does benzene undergo?
electrophilic substitution
naming aromatic compounds- sufffix
benzene ring is often considered to be the parent chain.
Alkyl groups (e.g. -CH3, -C2H5), halogens (F, Cl, Br, I) and nitro groups are all prefixes to benzene.
naming aromatic compounds- suffix
when benzene ring attached to alkyl chain with a functional group or to an alkyl chain with more than 7 carbons, benzene is considered the substituent.
instead of benzene, the prefix phenyl is used in the name
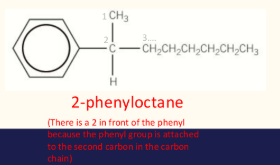
naming aromatic compounds- exceptions
Benzoic acid (C6H5COOH)
Phenylamine (C6H5NH2)
Benzaldehyde (C6H5CHO)
Phenol (C6H5OH)
electrophile
electron pair acceptor
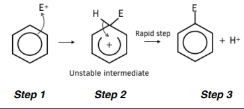
electrophilic substitution of benzene
replacing a hydrogen for another group
General equation: C6H6 + X+ → C6H5X + H+
X+ is the electrophile
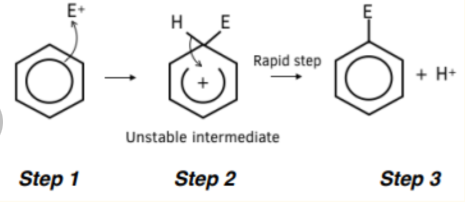
mechanism of the reaction of an alkene and bromine
The π-bond in the alkene contains localised electrons above and below the C=C plane. This is an area of high electron density
The localised electrons induce a dipole in the non-polar bromine molecule making one bromine slightly positive and the other slightly negative
The slightly positive bromine atom enables the bromine to act as an electrophile
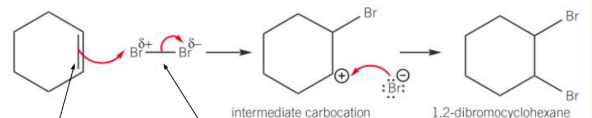
Comparing the Reactivity of Alkenes with Arenes
Because benzene has delocalised electrons above and below the plane of the carbon atoms in the ring structure. There is less electron density around around 2 carbon atoms in a benzene ring than round a C=C double bond in an alkene.
synthetic pathways- aromatic compounds
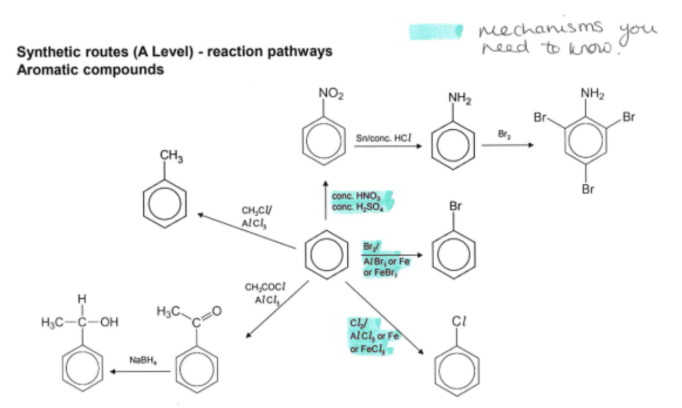
electrophilic substitution reactions of benzene
Nitration
Halogenation
Alkylation
Acylation
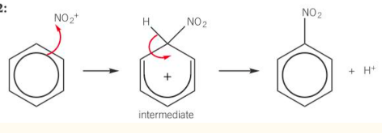
nitration of benzene
Reagent: conc. nitric acid (HNO3)
Catalyst: conc. sulfuric acid (H2SO4)
Conditions: 50-55 °C (if higher multiple substitutions occur)
electrophile is the nitronium ion (NO2+)
- H2SO4 + HNO3 → HSO4– + NO2+ + H2Oregeneration of catalyst
- H+ + HSO4- → H2SO4
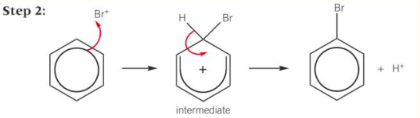
halogenation of benzene
only react if halogen carrier is present
- e.g. AIX3, FeX3electrophile formation: Br2 + FeBr3 → FeBr4- + Br+
catalyst reformation: H+ + FeBr4– → FeBr3 + HBr
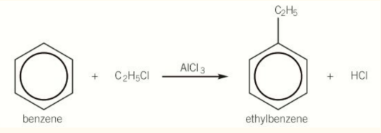
alkylation of benzene
It is a similar reaction mechanism as halogenation, using a halogen carrier (AlCl3) as a catalyst.
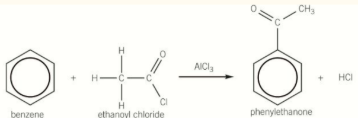
Acylation of benzene
uses halogen carrier AICl3 as catalyst
phenols
aromatic compounds that have hydroxyl group (-OH) attached directly to the ring
hydroxyl always takes position number 1
C6H5OH
phenols as weak acid
C6H5OH ⇌ C6H5O– + H+
e.g. Hydroxides: C6H5 + NaOH -> C6H5O-Na+ + H2O
p-orbital overlap into the ring weakens the O-H bond so phenol can donate H+ and form stable phenoxide ions

reactivity of phenol
Phenol is more acidic than alcohols but less acidic than carboxylic acids (can compare Ka)
Ethanol does not react with either sodium hydroxide (strong base) or sodium carbonate (weak base)
Phenol reacts with sodium hydroxide but not sodium carbonate
Carboxylic acids react with both sodium hydroxide and sodium carbonate
bromination of phenol
forms a white precipitate (orange — colourless)
does not need a halogen carrier (e.g. AlBr3)
carried out at room temperature
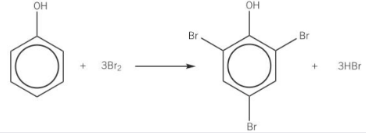
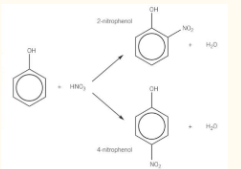
nitration of phenol
dilute nitric acid at room temperature
mixture of 2-nitrophenol and 4-nitrophenol is formed
phenol more reactive than benzene?
The oxygen atom bonded to the benzene has two lone pairs of electrons in p-orbitals.
As the ring is electron deficient, one of the p-orbitals overlaps into the ring structure to become delocalised.
Thus increasing the ring’s electron density, which attracts electrophiles more strongly than benzene
phenol has a higher electron density so can polarise more readily
do directing groups lesson
make flAshcards icba
what does a carbonyl contain?
C = O functional group
list the carbonyls
aldehyde, ketone and carboxylic acid
where is the carbonyl functional group found on an aldehyde?
at the end of carbon chain- CHO
where is the carbonyl functional group found on an ketone?
any carbon thats not the end- CO
whats the ending for aldehyde, ketone?
-al
-one
do carbonyls and alkenes reacts in the same way and why?
no, because C=O is polar whereas C=C is not
carbonyls react with nucleophiles – nucleophiles are attracted to the slight positive charge on the carbon.
nucleophiles
electron pair donor
oxidation of aldehydes form what and with what?
carboxylic acids
reflux with Cr2O7^2–/H^+ (i.e. K2Cr2O7/H2SO4)
how to write equation of oxidation of aldehydes e.g. butanal?
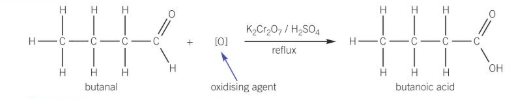
what does the oxidation of ketones form?
it can’t undergo oxidation reactions- has lack of reactivity
what’s the difference in the type of reaction that carbonyls and alkenes undergo?
alkenes undergo electrophilic addition
carbonyls undergo nucleophilic addition
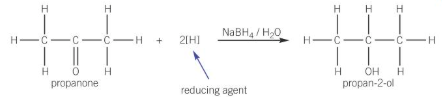
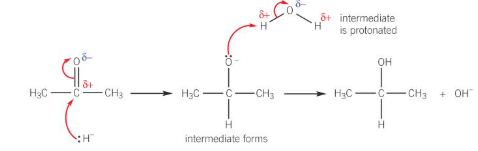
reactions of carbonyl compounds with NaBH4
NaBH4 acts as an reducing agent to reduce aldehyde and ketones into alcohols
warmed with the reducing agent in aqueous solution
reaction of aldehyde with NaBH4 e.g. butanal
reduced to primary alcohols
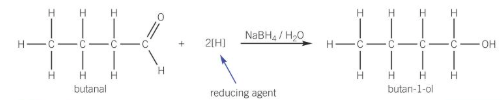
reaction of ketones with NaBH4 e.g. propanone
reduced to secondary alcohols
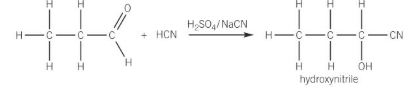
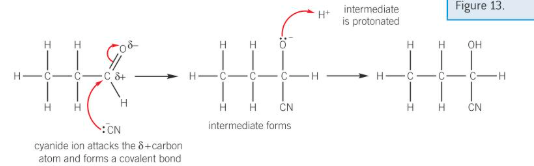
reactions of carbonyl compounds with HCN
form hydroxynitriles
using e.g. NaCN/H2SO4
increases the length of the carbon chain
reaction of aldehyde with HCN e.g. propanal
contains 2 functional groups
hydroxyl group (-OH)
nitrile group (C≡N)
mechanism for reaction between carbonyls and NaBH4
mechanism for reaction between carbonyls and NaCN/H^+
Testing for aldehydes and ketones
Add 2,4- DNP
If an aldehyde or ketone is present an orange precipitate will form.
identifying aldehydes and ketones
Using melting point- add 2,4 –DNP
orange precipitate forms
separate the solid from the solution
recrystallise the solid to form pure sample
the melting point is measured
compare to a database of known values
distinguishing between aldehydes and ketones
Add Tollens reagent (silver nitrate in aqueous ammonia) in a water bath at 50 degrees celsius
Silver mirror produced if an aldehyde is present
equation for aldehyde and tollens reagent
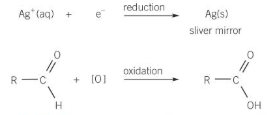
why can’t a ketone form a silver mirror with tollens reagent?
Silver ions are reduced to silver oxidising the aldehyde to a carboxylic acid. A ketone cannot be oxidised further.
carboxylic acid functional group
carbonyl group
hydroxyl group
solubility of carboxylic acids
the C=O and O-H bonds in carboxylic acid are polar
this allows carboxylic acids to form hydrogen bonds with water
making it soluble
diagram of why carboxylic acids are soluble
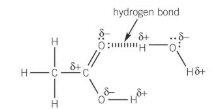
what happens to the solubility of carboxylic acids as chain length increases?
Solubility decreases as chain length increases because the carbon chain is non-polar and cannot form hydrogen bonds with water
strength of carboxylic acids
HCOOH (aq) ⇌ H^+ (aq) + HCOO^- (aq)
weak acids as they only partially dissociate in water
acid reactions of carboxylic acids
redox reactions with metals
neutralisation reactions with bases (alkalis, metal oxides, and carbonates)
carboxylic acids form carboxylate salts
carboxylate ions
Redox reactions of carboxylic acids + metal
Carboxylic acid (aq) + metal (s) 🡪 Carboxylate salt (aq) + hydrogen (g)
observations
Effervescence as hydrogen gas is evolved.
Metal disappearing as insoluble Mg reacts and forms the soluble salt
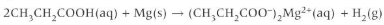
carboxylic acid + metal oxides
Carboxylic acids + metal oxide 🡪 carboxylate salt + water
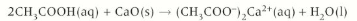
carboxylic acid + metal hydroxides
Carboxylic acids + metal hydroxide 🡪 carboxylate salt + water
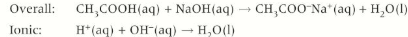
carboxylic acid + metal carbonate
Carboxylic acids + metal carbonate 🡪 salt + water + carbon dioxide
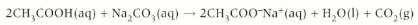
testing for carboxylic acids/carboxyl group
the neutralisation reactions between carboxylic acids and carbonates (e.g sodium carbonate) are important
carboxylic acids are the only common organic compounds sufficiently acidic to react with carbonates
Observation: FIZZING (not gas is formed)
derivatives of carboxylic acids
can be hydrolysed to form carboxylic acids
have a common sequence of atoms in their structureknown as acyl group
examples of carboxylic acids
Esters, Acyl Chlorides, Acid anhydrides
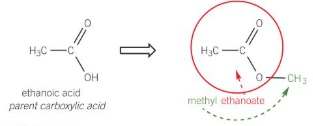
esters structure
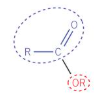
esters naming
remove the -oic acid suffix from parent carboxylic acid and replace with -oate
alkyl chain attached to oxygen atom of the COO group is then added as the first word in name
acyl chlorides structure
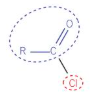
acyl chlorides naming
remove -oic acid suffix from parent carboxylic acid and replace with -oyl chloride
Acid anhydrides structure
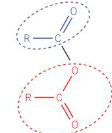
Acid anhydrides naming
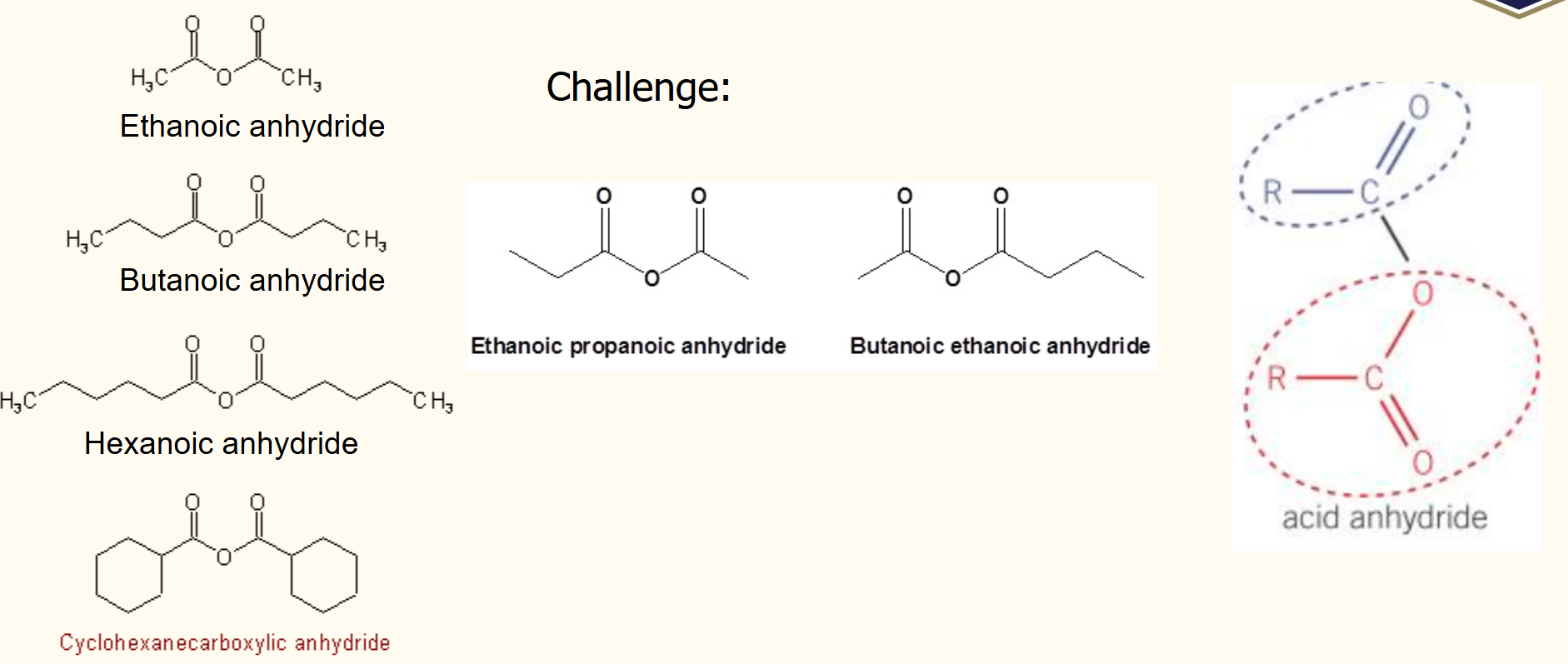
Acid anhydrides formation
removal of water from 2 carboxylic acids
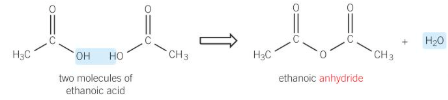
esterification
reaction of an alcohol with a carboxylic acid to form an ester
warmed with a carboxylic acid with small amount of conc. sulfuric acid (acts as a catalyst)
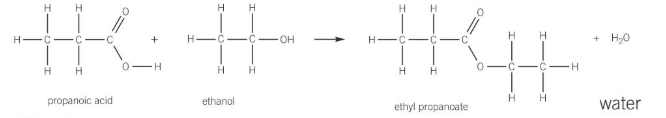
hydrolysis of esters
can be hydrolysed by either an aqueous acid or alkali
acid hydrolysis of esters
reverse of esterification- forms carboxylic acid and alcohol
ester heated under reflux with dilute aqueous acid
ester broken down by water, with acid acting as a catalyst
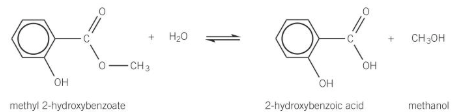
alkaline hydrolysis of esters
ester heated under reflux with aqueous hydroxide ions
forms carboxylate ion and an alcohol
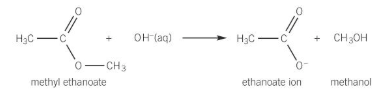
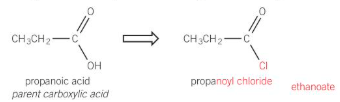
formation of acyl chloride
when SOCl2 reacts with a carboxylic acid
- other products SO2 and HCl are evolved as gases leaving just the acyl chloride
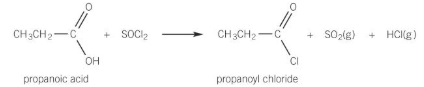
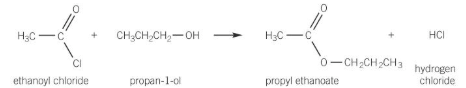
reactions of acyl chlorides
very reactive
react with nucleophiles by losing chloride ions whilst retaining C=O
Acyl chloride + Alcohol
forms ester and HCl
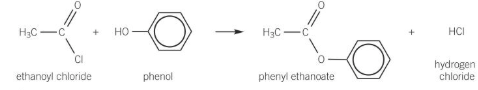
Acyl chloride + Phenol
Acyl chloride + water
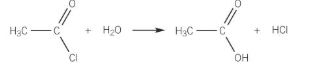
ammonia formula
NH3
ammonium structure
NH4^+
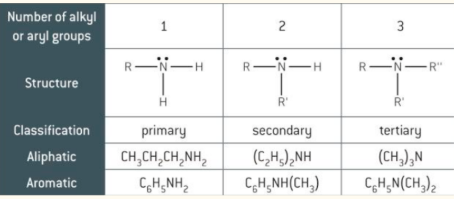
amine structures e.g. primary, secondary, tertiary
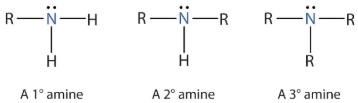
amine structures e.g. primary, secondary, tertiary
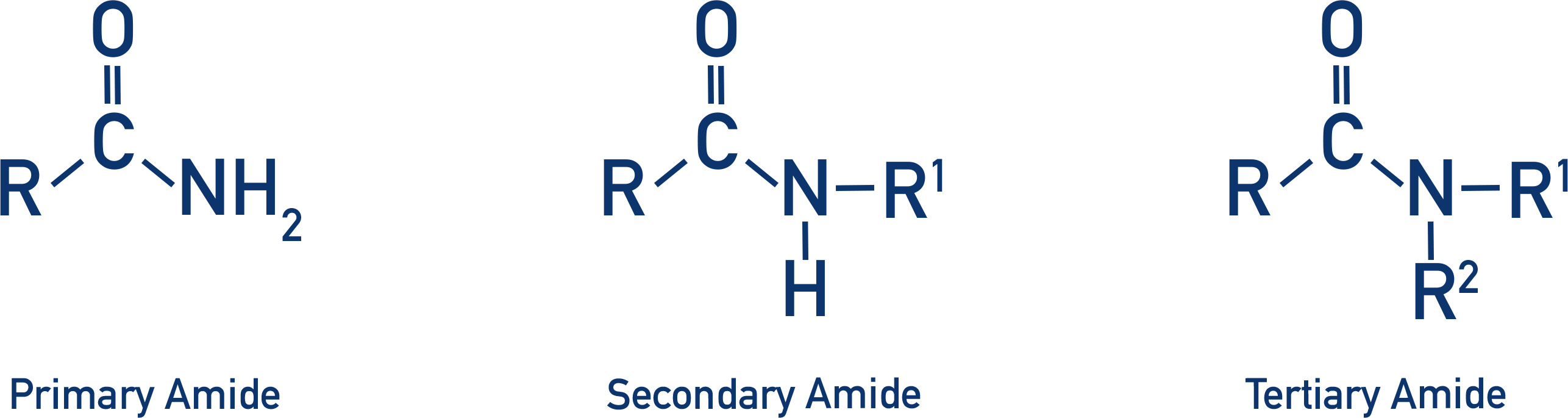
acyl chloride + ammonia
ammonia acts as a nucleophile
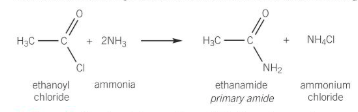
acyl chloride + primary amine
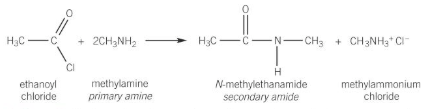
amines
Derived from ammonia
Replace hydrogen/s with an organic group
amines fall into different classes depending on how many of the hydrogen atoms are replaced
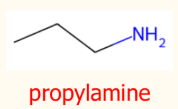
NH2 group is on the end of a chain:
Suffix: Amine
Prefix: alkyl chain
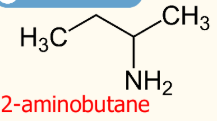
NH2 group is middle of the chain
Suffix: Alkane chain
Prefix: Amino
more than 1 group attached to the Nitrogen
Longest chain in front of amine
N in front of every other chain coming off N
Put in alphabetical order
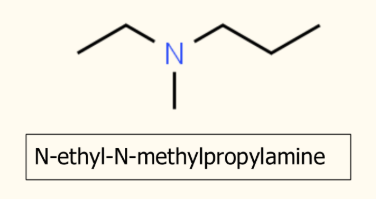
how do amines act as bases
ability to accept a proton (H+)
have a lone pair of electrons which can accept a proton to form a dative covalent bond
able to neutralise acids to make salts
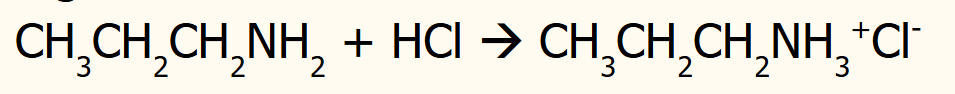
preparation of amines-primary
can act as a nucleophile in a nucleophilic substitution reaction with a haloalkane
Conditions: excess ammonia (prevents further substitution of the amine), ethanol (solvent)

preparation of amines- secondary and tertiary
The product CH3CH2NH2 still contains a lone pair of electrons on nitrogen that can react further with a haloalkane to form a secondary amine.
RCl + RNH2 🡪 R2NH2+Cl-
- e.g. CH3CH2Cl + CH3CH2NH2 🡪 (CH3CH2)2NH2+Cl-R2NH2+Cl- + NaOH 🡪 R2NH +NaCl + H2O
- e.g. (CH3CH2)2NH2+Cl- + NaOH 🡪 (CH3CH2)2NH +NaCl + H2O
Tertiary amines can then be produced by a further reaction of a secondary amine with a haloalkane.
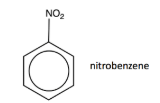
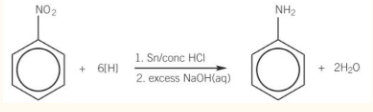
preparation of aromatic amines
phenylamine by reduction of nitrobenzene
nitrobenzene heated under reflux with tin and hydrochloric acid
then reacted with excess sodium hydroxide
tin and hydrochloric acid act as a reducing agent.
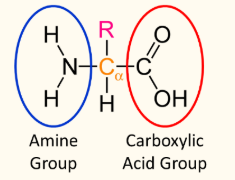
amino acids
contain both an amine group (-NH2) and a carboxylic acid (-COOH) functional groups
α-amino acids contain both an amine and a carboxyl group that are separated by one carbon atom
general formula: RCH(NH2)COOH