Orgo Prelim #2 Material
1/11
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
12 Terms
more stable formation (E vs. Z)
E, larger groups are more spread apart
Addition of Halides
H goes on C with a smaller number of alkyl substituents
X bonds to C with more alkyl subsituents
if there is excess and an alkyne is present, the 2 X will go on the SAME carbon (more subsituents)
what is an intermediate carbon?
carbocation (2 prime is carbon bonded to 2 carbons). A tertiary carbocation is more stable (groups help with the positive charge)
Hydrations of Alkenes
Addition of an OH (often shown as H3O+)
alkeenes → alcohols
Pt/C catalyst (or Pd/C)
alkene to alkane adding 2 hydrogens
lindlar cataliyst
Alkyne → cis alkene
ALWAYS cis formation
hydroboration-oxidation
BH3. The B goes on the less substituted carbon (less EN than H) and is then later substituded by an OH
BH3/THF
H2O2, -OH
oximercuration-reduction
OH on the MORE substituted C.
Hg(OAc)2/H2O
NaBH4/NaOH
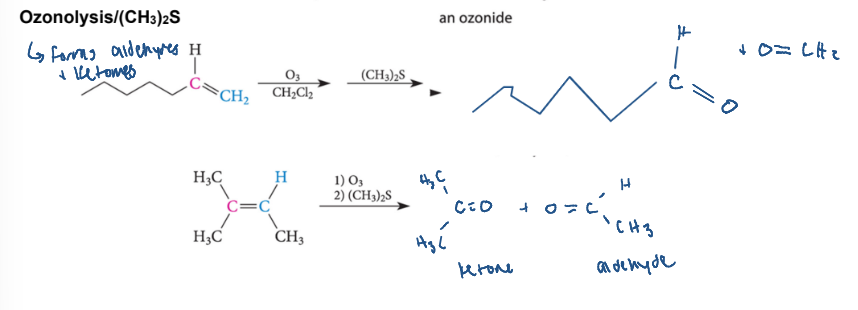
ozonolysis to form aldehyde + ketone
forms a aldehyde and a ketone
O3
(CH3)2S
ozonolysis to form carboxylic acid
H2O2 + H2O causes aldehydes to become carboxylic acids
hydration of alkynes
alkynes form ketones
hg2+, H2SO4
hydroboration with alkynes
Bh3 -THF
H2O2, -OH
alkynes → ketone