Introduction to Organic Chemistry
1/9
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
10 Terms
Describe bonding in Carbon Compounds?
In all stable carbon compound, carbon forms four covalent bonds and has eight electrons in its outer shell. These can be used to form rings of carbon or chains.
It can do this by forming 4 single bonds (Methane), forming two single bonds and one double bond (Ethene) or by forming one single bond and one triple bond (Ethyne)
The carbon-carbon bond is relatively strong and non-polar.
What are the different ways of writing and representing Organic Compounds?
Empirical Formula - The simplest whole number ratio of atoms of each element in a compound
Molecular Formula - The true number of atoms of each element in a compound
General Formula - All members of a homologous organic series follow its general formula (CnH2n+2)
Structural Formula - A representation of the unique arrangement of atoms in a molecule (CH3CH3). Branches in the carbon chain are shown in brackets (CH3CH(CH3)CH3)
Displayed formula - A graphic representation showing every atom and bond in a molecule
Skeletal Formula - A simplified graphic representation that shows only the bonds and any non-carbon atoms in a compound. The vertices are carbon atoms and hydrogen is assumed to be bonded unless stated otherwise (See Image)
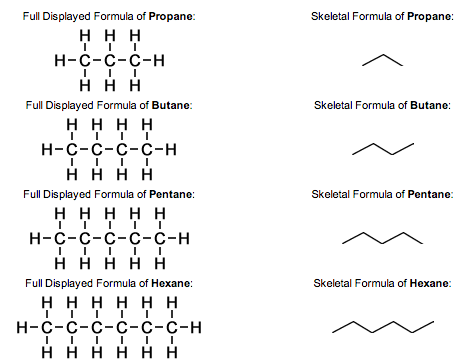
What is a Homologous Series?
A set of organic compounds with the same functional group and general formula but different carbon chain length. They react similarly and each member differs by CH2.
The length of the chain affects properties like melting point, boiling point and solubility.
Melting and Boiling points increase by a small amount as the number of carbon atoms in the chain increases due to the intermolecular forces increasing.
Chain branching also reduces melting point because the molecules pack together less well.
What is a Functional Group?
An atom or a group of atoms in an organic molecule which is responsible for the characteristic reactions of that molecule.
What are Reaction Mechanisms?
The series of simple steps that lead from reactants to products in a chemical reaction.
Curly arrows are used to show the movement of electrons. The arrow usually starts from a lone pair of electrons or from a covalent bond to a positively charged area of a molecule to form a new bond.
Free Radicals can also be shown when a covalent bond breaks away so that one electron goes to each atom in the original bond, resulting in the unpaired electrons making the atom extremely reactive.
What are the steps to naming a Organic Compound?
Root - The systematic name has a root which tells us the longest unbranched hydrocarbon chain or ring. Meth = 1, Eth = 2, Prop = 3, But = 4, Pent = 5, Hex = 6.
Suffix - The suffix indicates the functional group of the compound. Year 1 suffixes shown at bottom.
Chain/Position Isomers - The labelling of a side chain or functional group relative to the main chain. The number indicates the position of the functional group or branch, starting from either end to the nearest functional group. The substituents are listed in alphabetical order with their location numbers.
If you have more than one substituent you can use prefixes like di- , tri- and tetra-.
What is an Isomer?
Isomers are molecules that have the same molecular formula but have a different structural arrangement of atoms.
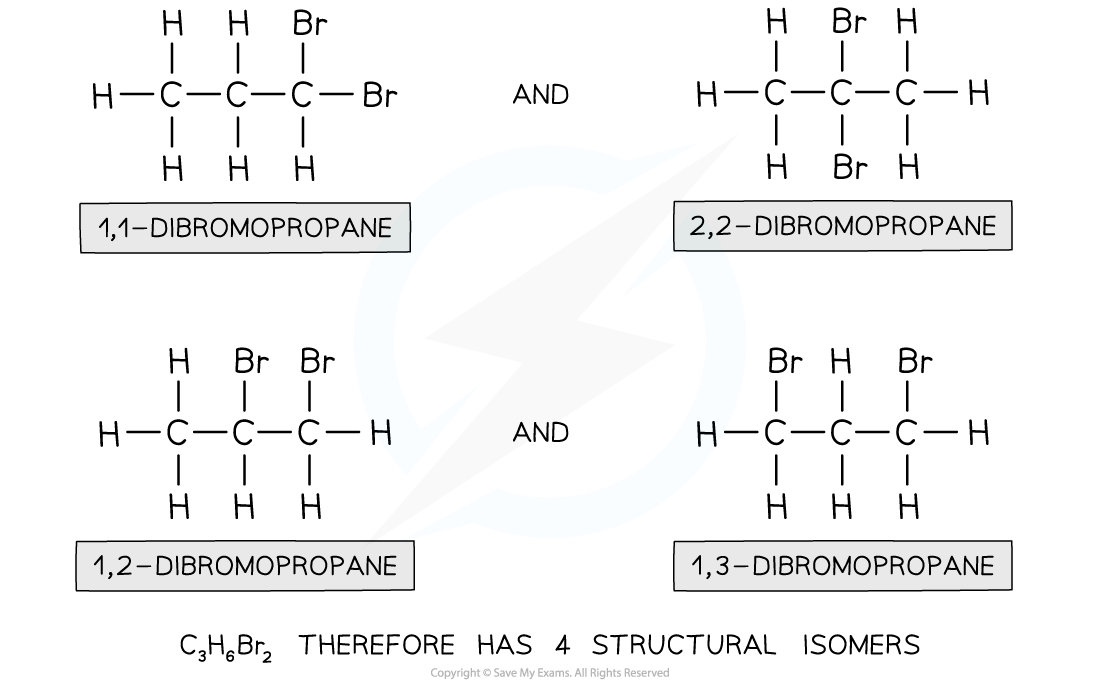
What are Structural Isomers?
A structural Isomer is a isomer with the same molecular formula but different structural formula. There are three sub-divisions: Positional, Functional and Chain Isomerism.
Positional Isomerism - Isomers with the same functional groups attached to the main chain at different points. C3H7Cl could represent 1-Chloropropane or 2-Chloropropane.
Functional Group Isomerism - Isomers that contain different functional groups. C2H6O could represent Ethanol or Methoxymethane (A Ether).
Chain Isomerism - Isomers that have a different arrangement of the hydrocarbon cain. C4H9OH could represent Butan-1-ol or 2-methyl-propan-1-ol.
What is Stereoisomerism?
Stereoisomerism is where molecules have the same molecular formula and the same structure but a different position of atoms in space. The two types are E-Z Isomerism and Optical Isomerism.
E-Z Isomerism - A type of stereoisomerism which tells us about the positions of substituents at either side of a carbon-carbon double bond. Two substituents on the same side begin with Z- and on the opposite sides begin with E- .
Substituted groups joined by a single bind can rotate around it so there are no isomers, but there is no rotation around a double bond so Z- and E- isomers are separate compounds.
Optical Isomerism is not needed in Year 1.
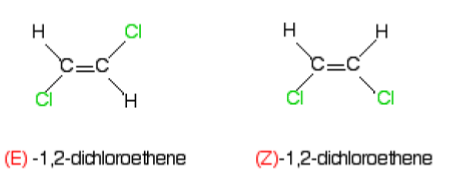
What are the Cahn-Ingold-Prelog (CIP) Priority Rules?
The CIP priority rules determine whether a compound has E or Z Isomerism. The first atom which is directly bonded to the carbon atom with the highest atomic number is given priority over the other atom bonded to it. If both the atoms with the highest atomic number are on the same side (Both top or bottom) then it is Z-, if not it is E-.