Physiology Exam One
1/84
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
85 Terms
Cell
Cell: Basic structural and functional units of life. 50-100 trillion cells in the human body. 3 main regions of the cell: 1. Plasma membrane 2. Cytoplasm 3. Nucleus.
Plasma membrane: thin, flexible membrane of the cell, separates intracellular from extracellular compartment. Plasma membrane is a double layer of lipids (phospholipids, cholesterol, and glycolipids) with embedded proteins. Phospholipids are most abundant in the plasma membrane. The heads are hydrophilic and lie along the inner and outer face of the membrane. The tails are hydrophobic and line up in the center of the membrane.
Membrane Proteins
2 types of membrane proteins are integral and peripheral.
Integral proteins: most abundant, most extend entirely through the membrane (transmembrane), but some can protrude through one side, and can act as a receptor. If there is a mutation to an integral protein and it changes the structure, it can lead to hormonal disorders (like type 2 diabetes)
Peripheral proteins: mainly on the cytoplasmic side by a network of filaments. Glycocalyx: short chain of carbohydrates (sugars) projected from the external surfaces of glycoproteins and glycolipids, used for cell-to-cell binding and recognition, produced by corneal epithelial surface cells.
Functions of the plasma membrane
1. Serves as an external cell barrier against substances and forces
2. Externally facing proteins act as receptors (hormones, neurotransmitters) and in cell-to-cell recognition
3. Transport of substances in and out of the cell (selectively permeable)
Passive, active, and vesicular transports
Passive transport: substances can pass freely through the lipid bilayer and down the concentration gradient, no ATP needed (more concentrated -> less).
Diffusion: small, uncharged molecules (O, CO2, fat soluble molecules).
Active transport: movement against the concentration gradient from low to high concentration, requires ATP, usually larger water-soluble or charged molecules transported by a pump and involve integral proteins (ex. Glucose, A.A., and ions).
Vesicular transport: type of active process, passes large particles and macromolecules, two types: exocytosis and endocytosis
Exocytosis
Vessicles will fuse with the plasma membrane and release their contents to the outside of the cell. Proteins from the vesicle membrane (vSNAREs) bind with plasma membrane proteins (tSNAREs), causing the lipid layers of the vesicle and cell membrane to join together. Releases contents from the cell into the blood.
Endocytosis
Brings large molecules into the cell by enclosing them and forming a cytoplasmic vesicle. Clathrin protein (on the cytoplasmic side) deforms the membrane and allows infolding to occur.
Three types of endocytosis: phagocytosis, pinocytosis, and receptor mediated endocytosis.
Phagocytosis, Pinocytosis, and Receptor mediated endocytosis
Phagocytosis (cell eating): plasma membrane forms pseudopodes and englufs large molecules (like bacteria or cellular debris), forming a membranous vesicle called a phagosome. The phagosome will fuse to the lysosome, leading to enzymatic breakdown of phagosomal contents.
Pinocytosis (cell drinking): small infolding of the plasma membrane surrounds a small amount of extracellular fluid containing dissolved molecules (ex. Cells lining the small intestine absorb nutrients).
Receptor mediated endocytosis: selective, specific molecules (insulin, hormones, enzymes, LDL) are brought into the cell by attaching to a receptor and being transported in a protein-coated vesicle. The vesicle binds to the lysosome to release contents and the receptor is recycled. When the LDL receptor has a mutation and cholesterol cannot enter the cell (or ovaries or testicles, as cholesterol is the precursor to sex hormones), cholesterol increases in the blood
Familial Hypercholesterolemia
Inherited disease. Individuals lack the receptor that binds to cholesterol binding LDL. Cholesterol cannot enter the cell and builds up in the blood. This can cause hypercholesterolemia and atherosclerosis -> stroke or myocardial infarction.
Cytoplasm
Cellular region between the nucleus and plasma membrane. Contains cytosol (viscous fluid containing water, ions, and enzymes, inclusions for stored nutrients/pigments, and organelles)
Ribosomes
Dark staining granule with no membrane, site of protein production. Two subunits: protein and ribosomal RNA. Free ribosomes produce proteins used in the cytosol. Ribosomes attached to the rough ER make proteins used for the cell membrane or are exported outside of the cell. A.A. on the ribosome are linked together to form protein (in translation, dictated by DNA of the nucleus. These instructions are carried to the ribosomes by mRNA)
Rough and Smooth ER
Rough ER: ribosome-studded system of membrane-walled envelopes in the cytosol (called cisternae). Makes proteins that enter the cisternae and are secreted by the cell in vesicles. Also makes proteins for the cell membrane.
Smooth ER: network of membranous system of sacs and tubules in the cytosol. Involved in the synthesis of lipids and steroids, lipid metabolism, and drug detoxification.
Golgi Apparatus
Stack of 3-10 disc-shaped envelopes or cisternae that are bound by the membrane. Cisternae have cis (convex) and trans (concave) faces. Sorts the products of rER and packs them into membrane lined vesicles + transports them. Secretory granules and lysosomes arise from the golgi apparatus.
Mitochondria, function (ATP production), Chemiosmosis
Rod-like organelles covered by two membranes in the cytoplasm. The inner membrane is folded into projections (cristae). Main energy generator of the cell and site of ATP production. Chemiosmosis occurs in the inner membrane of the mitochondria using a proton gradient across a membrane to make ATP.
Lysosomes, Tay-Sachs disease
Lysosomes are spherical membrane-walled sacs containing digestive enzymes (acid hydrolases). Site of intracellular digestion for deteriorated organelles and substances brought into the cell via a vesicle. Fuse with the phagosome and empty their enzymes into the phagosome to break down the contents (phagocytic cells have many lysosomes).
Tay-Sachs disease: inhereted, infants lack the enzyme to break down certain glycolipids. Glycolipids accumulate in the cell membrane (on neurons), resulting in mental retardation, blindness, spastic movements, and death within 1.5 years of birth.
Gaucher’s Disease
Lack of glucocerebrosidase enzyme causes harmful substances to build up in the liver, spleen, bones, and bone marrow. Substances prevent cells from working properly. Three main types;
Type 1: most common, bone disease/anemia/enlarged spleen/thrombocytopenia, in children and adults.
Type 2: begins in infancy with severe neurological involvement, rapid+early death.
Type 3: Liver/spleen/brain problems, patients may live into adulthood. Symptoms vary on type but generally include bone pain/fractures, enlarged spleen + liver, lung disease, and seizures.
Peroxisomes
Membrane-walled, enzyme-containing sacs (oxidase and catalse). Oxidase uses oxygen to neutralize aggressively reactive substances (free radicals) by converting them to hydrogen peroxide. Hydrogen peroxide is converted to water and oxygen by catalse, which break down poisons like alcohol, phenol, and formaldehydes that enter the body. The liver and kidney have a lot of peroxisomes.
Microtubules, Microfilaments, and Intermediate functions (only function)
These are the three types of cytoskeletons that run throughout the cytosol and support the cellular structure and generate movements of the cell.
Microtubules: gives the cell its shape and organizes the distribution and transport of various organelles within the cytoplasm.
Microfilaments: made of actin, involved in muscle contractions and other types of cellular movement (amoeboid movements and extension of pseudopods).
Intermediate filaments: resist tension placed on the cell.
Centrosomes and Centrisoles
Centrosome: spherical structure near the nucleus, has an outer cloud of protein (matrix) and an inner pair of centrioles. Matrix protein helps with elongation of microtubules and mitotic spindle of microtubules radiates from it in dividing cells.
Centrioles: core of the centrosome, pairs of cylindrical bodies perpendicular to one another, each with nine triplets of microtubules. They organize a microtubule network during mitosis to form the spindle and asters.
Main parts of the nucleus
The nucleus is the control center of the cell and contains DNA, which provides instructions for protein synthesis. Most cells have one nucleus, some have multiple (skeletal muscle), and red blood cells have none. There are 3 main parts to the nucleus;
1. Nuclear envelope: Surrounds the nucleus, has pores and is continuous with the ER
2. Chromatin and chromosomes: chromatin is the thread-like material in the nucleus composed of DNA and histone proteins. Chromosomes contain a single, long piece of DNA (46 in a typical human cell) and has chromatin distributed throughout.
3. Nucleoli: dark staining body within the nucleus that contains parts of the chromosomes and is the cell’s ribosome producing center (code for rRNA)
DNA
Double helix chains of nucleotide molecules. Nucleotides have sugar, phosphate, and one of the four bases: thymine, adenine, cytosine, and guanine (bind to hold the DNA together like a ladder). DNA wraps around clusters of eight spherical proteins (histones) which regulate gene expression and transcription. Each cluster of DNA and histones is called a nucleosome. Zygote has 46 single chromosomes, half from mom and half from dad.
Cell Life Cycle
Cell life cycle is a series of changes a cell experiences from the time it forms until it reproduces itself. Two major time periods:
1. Interphase: cell grows and carries out its usual activities
2. Cell division (mitotic phase): cell divides into 2, essential for growth and repair of the body.
Interphase
Non-dividing phase of the cell cycle, three subphases;
1. G1 (gap 1): cells are active and grow vigorously, centrioles start to replicate.
2. S (synthetic): DNA replicates itself for two daughter cells with the same genetic material
3. G2 (gap 2): enzymes needed for cell division are synthesized, centrioles finish replication and the cell gets ready to divide.
Mitosis (4 parts)
Four phases include prophase, metaphase, anaphase, and telophase.
Prophase: asters (stars) are formed (microtubule arrays that extend from the centrosome), chromosomes are formed (each chromosome has 2 identical chromatin threads called chromatids, chromatids are held together by a centromere and protein complex called cohesin), nucleoli disappear, centriole pairs separate, nuclear envelope fragments, microtubules disassemble and are newly assembled to form mitotic spindles (push centrioles to poles of the cell).
Metaphase: Chromosomes cluster at the center of the cell to form a metaphase plate, separase starts to separate the chromatids. All chromosomes in one line like a soldier.
Anaphase: V-shaped chromatids are pulled apart by kinetochore spindles, spindles push apart to elongate the cell.
Telophase: chromosomes uncoil and resume extension, nuclear evelope forms by the rER, nucleoli appear. Cells are then separated through cytokinesis.
Meiosis
Cell division that results in the formation of gametes, two divisions -> 4 gametes. Each gamete has 23 chromosomes (half normal) and 1N DNA (half).
Meiosis 1 has large segments of DNA exchanged and the centromeres do NOT split. Results in 2 secondary gametocytes with 23 duplicated chromosomes and 2N DNA.
Meiosis 2 does not have crossing over but the centromeres DO split, resulting in 4 gametes with 23 single chromosomes and 1N DNA. Abnormal disjunction step in Meiosis 2 leads to genetic disorder - called aneuploidy. (ex. down syndrome - chromosome 21)
Aneuploidy and syndromes
Aneuploidy is an abnormal number of chromosomes, can be trisomy and monosomy, Down syndrome is trisomy 21. Klinefelter syndrome (XXY). Turner syndrome (XO) is monosomy,
Gametes definition
Contain 23 single chromosomes and 1N DNA. Called a “haploid” due to 23 chromosomes. Female gametes have the X chromosome and male can have X or Y.
Aging, Mitochondrial theory
Aging: complex and may involve cell damage due to free radicals as a result of normal cell metabolism or cell injury due to radiation/chemical pollutants.
Mitochondrial theory of aging: decreased energy production by radical-damaged mitochondria which weakens and ages the cell. Vitamins C and E act as antioxidants and prevent excessive production of free radicals. Same for lowering caloric intake to lower metabolic rate and slow aging.
Genetic theory of aging: proposes aging is programmed into our genes.
Apoptosis
Method of cell removal where cells are removed from tissues in an orderly fashion as a part of normal maintenance or during development. Cells that undergo apoptosis usually have chromatin condensation, breaking up of the nucleus/plasma membrane, cell shrinks, cell fragmented into apoptotic bodies. Certain cytokines, like tumor necrosis factor (TNF), can activate caspases 3 and 9 that degrade proteins in the nucleus and cytoplasm leading to the morphological characteristics of apoptosis. Defects in apoptosis lead to many major diseases: too much apoptosis causes nerve damage and can lead to Alzhiemers and stroke, too little apoptosis linked to cancer and other autoimmune diseases.
Benign / Malignant
Cancer comes from a cell mass that divides and multiplies abnormally, called a neoplasm. This neoplasm can be benign or malignant.
Benign: remains compacted, often encapsulated, grows slowly, seldom kills the host.
Malignant: grows rapidly, immature cells invade their surroundings/surrounding cells, gives metastasis (invading of other tissue) through the blood or lymphatics.
Oncogenes
Oncogenes are the result of mutations of certain regulatory genes, called protooncogenes, which are normally supposed to stimulate/inhibit cell proliferation and development. Genetic accidents or viruses can lead to formation of oncogenes. Oncogenes dominate the normal alleles, causing deregulation of cell division (cancer). Oncogenes can cause bladder cancer and myelogenous leukemia
Tissues, nervous tissue, and epithelial tissue (function)
Tissues are a collection of structurally similar cells with related function. There are 4 main types of tissues.
Muscle tissue: contractile tissue of the body derived from the mesodermal layer of embryonic germ cells. Produces force and causes motion, and can be skeletal, smooth, or cardiac. Skeletal muscle tissue is attached to the skeleton and applies force to bones and joints, smooth muscle tissue is found within the walls of organs and other structures, and cardiac muscle tissue is involuntary and only found in the heart.
Nervous tissue: specialized, reacts to stimuli and conducts impulses. Found in the brain, spinal cord, and peripheral nerves, and the tissue is made of neurons. Neurons are easily stimulated and transmit impulses rapidly. A nerve is made from many neurons bound together by connective tissue. Dense connective tissue, called the epineurium, surrounds the nerve. This epineurium penetrates the nerve to form the perineurium (surrounds the bundles of nerve bundles), and there are blood vessels in the epineurium. The endoneurium consists of a thin layer of loose connective tissue and surrounds individual nerve fibers.
Epithelial tissue: many functions; 1. Protection (underlying tissues from invaders, loss of water, etc) 2. Sensation Sensory stimuli (penetrate specialized epithelial cells containing nerve endings) 3. Secretion in glands (secretes specific chemical substances, like enzymes/hormones) 4. Absorption (absorb nutrients from the digestion of food) 5. Excretion (excrete waste products from the body and reabsorb needed materials from the urine) 6. Diffusion (diffusion of gases, liquids, and nutrients) 7. Cleaning (removes dust particles and foreign bodies) 8. Reduces friction (from the blood and walls of blood vessels). Found in every part of the body.
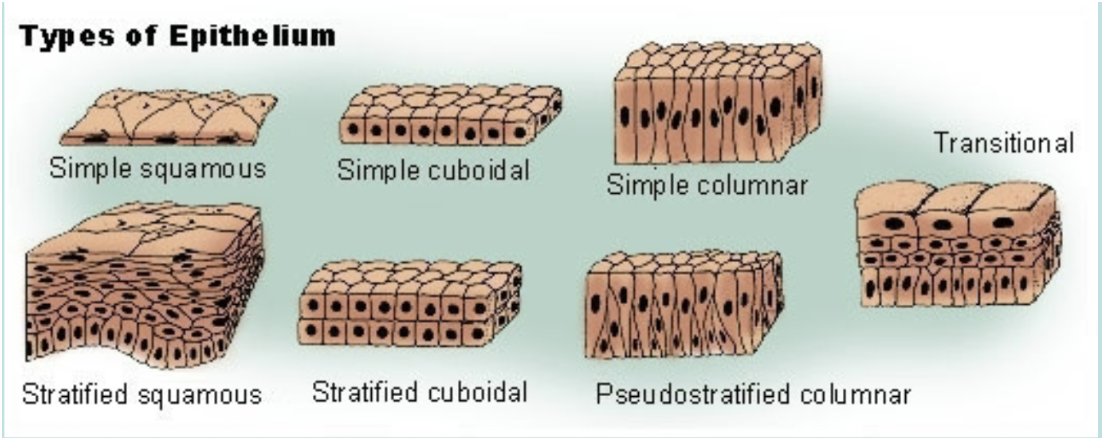
Epithelia cells (name, function, and location)
Epithelia are sheets of cells that cover body surfaces and cavities, glands are covered internally epithelial cells which function in secretion of their products. Epithelial cells are classified by their shape into three main categories: 1. Squamous (flat) 2. Cubodial (cube like) 3. Columnar (tall and rod-like). They can also be simple (one layer) or stratified (multiple layers, superficial layer is used to classify the layer, can withstand a large amount of stress, only one layer touches the basal lamina).
Pseudostratified with cilia: mainly used with pseudostratified columnar epithelium, there is only one layer of cells but the position of the nuclei gives the impression that it is stratified.
Simple squamous epithelia: line body cavities and capillaries to reduce friction, line the alveoli to facilitate gas exchange, gas/substances can easily diffuse and liquids can flow easily over them.
Simple cuboidal epithelia: function is for secretion and absorption, found in glands and lining of kidney tubules, as well as in the ducts of the glands. Constitute the germinal epithelium which produces female eggs and male sperm.
Simple columnar epithelia: forms the lining of the stomach and intestines, some are specialized for sensory reception (nose, ears, and taste buds), goblet cells are found between the columnar epithelial cells of the duodenum. Function to secrete mucous or slime, a lubricating substance.
Pseudostratified columnar epithelia: found in ducts of large glands, ciliated variety lines the trachea. Function for secretion of mucous.
Stratified cuboidal epithelia: function to protect the ducts of sweat glands and the male urethra.
Stratified columnar epithelia: function to protect and secrete, small amounts in male urethra and in large ducts of some glands.
Stratified squamous epithelium: found in the vagina.
Transitional epithelia: stretches readily and permits distension of urinary organ, lines the ureters, bladder, and part of the urethra.
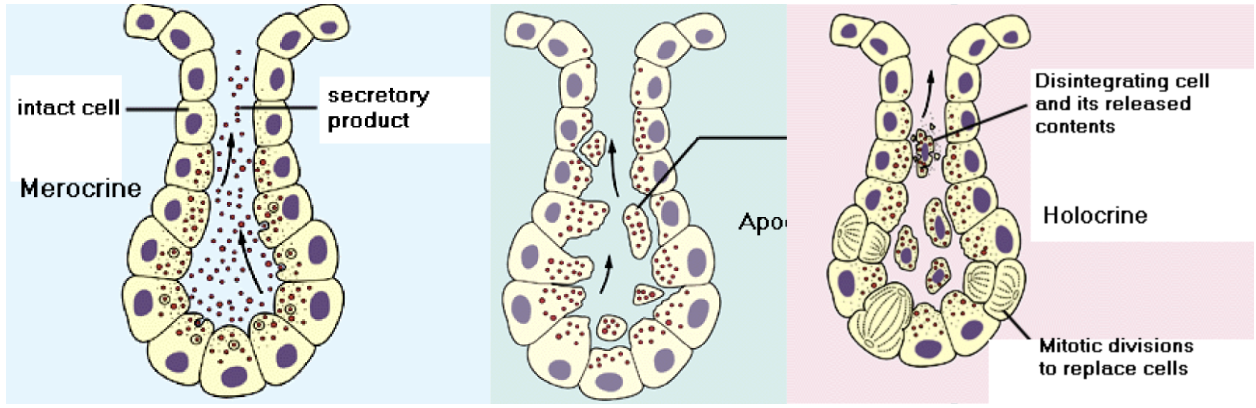
Glands: Exocrine glands (Merocine, apocrine, and holocrine; location and function)
Glands are classified as exocrine or endocrine. Exocrine are glands that retain ducts to body surfaces. Products of these cells collect in the duct of the gland and flow toward the surface to which the duct is in contact. Then they secrete their products onto body surfaces/cavities. Goblet cells are examples of mucus secreting unicellular exocrine glands. Multicellular exocrine glands are classified by the structure of their ducts as simple or compound and by the structure of their secretory units as tubular, alveolar, or tubuloalveolar. Modes of secretion for secretory cells to release their products into ducts are: merocrine, apocrine, and holocrine.
Merocrine: cells form membrane-bound secretory vesicles internal to the cell. These are moved to the apical surface where vesicles coalesce with the membrane in the apical surface to release the product. Most glands release their products this way.
Apocrine: the apical portions of cells are pinched off and lost during the secretory process. This results in a secretory product that contains a variety of molecular components, including those of the membrane. Mammary glands release their products in this manner.
Holocrine: involves death of the cell. The secretory cell is released as it breaks apart, the contents of the cell become the secretory product. Results in most complex secretory product. Some sweat glands in the axillae, public areas, and around the ateoli of breasts release in this manner, sebaceous glands are this type too.
Endocrine gland (such as Pancreas secretes 3 hormones - insulin, glucagon, and somatostatin)
Referred to as ‘ductless’ glands. Their product is released across the cell membrane into interstitial spaces around the cells. Diffusion of product into capillaries follows. They secrete hormones which enter circulation and reaches target tissue to have their effects (ex pancreas produces insulin and glucagon)
Cell junctions (name, structure, function)
Special cell junctions hold epithelial cells together and there are four types: tight junctions, adhering junctions, desmosomes, and gap junctions.
Tight junctions: aka zonula occludens, type of junctions complex. Formed by claudin and occludin proteins, joining the cytoskeletons of adjacent cells. They function to hold cells together, block movement of integral membrane proteins between apical and basolateral surfaces of the cell, preserve transcellular transport, and prevent passage of molecules and ions through space between cells.
Desmosomes: two disc-like plaques connected across intercellular space. Plaques of adjoining cells are joined by proteins called cadherins, proteins interdigitate into extracellular space. Intermediate filaments insert into plaques from the cytoplasmic side.
Gap junctions: passageway between two adjacent cells, they let small molecules move directly between neighboring cells (act as channel for ions). Cells are connected by hollow cylinders of protein.
Marfan Syndrome
An autosomal dominant genetic disorder of the connective tissue characterized by disproportionately long limbs, long thin fingers, tall stature, and a predisposition to cardiovascular abnormalities (those affecting the heart valves and aorta). Has been linked to a defect in the gene on chromosome 15 which encodes a glycoprotein called fibrillin-1. Fibrillin is essential for the formation of the elastic fibers found in connective tissue.
Ehlers-Danlos Syndrome
Group of rare genetic disorders affecting humans and domestic animals caused by a defect in collagen synthesis (collagen 1 and 3). Looks like hyperextension - extreme elasticity of the skin and flexible joints.
Body Fluids
67% of total weight is water within cells, in the intracellular compartment. Remaining 33% of body water comprises the extracellular compartment. 20% of this extracellular fluid is contained in vessels of cardiovascular system, where it comprises the fluid portion of the blood, or blood plasma. Extracellular space is a combination of blood vessels and interstitial space - collagen, polysaccharides, glycoproteins, and integrin. Blood transports oxygen from lungs to the body cells and CO2 from the body to the lungs. It also transports nutrients from food in the intestines to body cells along with nutrients between organs (like glucose from liver to brain or lactic acid from muscles to liver). The remaining 80% of the extracellular fluid is located outside of the vascular system and comprises tissue fluid, aka interstitial fluid.
Extracellular matrix, integrins
The extracellular environment fluids, as interstitial, or tissue, fluid, is within a matrix of glycoproteins and proteoglycans. It consists of protein fibers collagen and elastin. The fluid (derived from blood plasma) provides nutrients and regulatory molecules to the cells. The extracellular environment is supported by collagen and elastin protein fibers, which form the basal lamina below epithelial membranes.
Integrins: class of glycoproteins that extend from the cytoskeleton within a cell, through its plasma membrane, and into the extracellular matrix. By binding to components within the matrix, they serve as adhesion molecules between cells and extracellular matrix.
Passive, active (primary, secondary)
Passive transport: net movement of molecules and ions across a membrane from higher to lower concentration that does not require metabolic energy.
Active transport: new movement across a membrane that occurs against a concentration gradient and requires ATP.
Primary active transport: occurs against an electrochemical gradient (uphill), requires direct input of metabolic energy (ATP), is carrier-mediated and exhibits stereospecificity, saturation, and competition. Examples- Na+ and K+ (pump) in cells transports Na+ from intracellular to extracellular and K+ from extracellular to IC fluid. Ca2+ with ATPase in the sarcoplasmic reticulum or cell membrane. H+, K+ and ATPase in gastric parietal cells transports H+ into lumen of the stomach (inhibited by omeprazole - proton pump inhibitor). Oversecretion of H+ into lumen of stomach leads to irritation of stomach = gastritis.
Secondary active transport: transport of two or more molecules is coupled, one of the solutes (usually Na+) is transported downhill and provides energy for the uphill transport of the other solute, metabolic energy is not provided directly, solutes can move in same direction across the cell membrane (cotransport/symport), solutes can move in opposite directions across the cell membrane (countertransport/exchange/antiport). Sodium (Na) can transport potassium (K), chloride (Cl-), amino acid, phosphate, glucose, calcium (Ca2+)
Simple diffusion
Characteristics - only form of transport that is not carrier-mediated, occurs down an electrochemical gradient (downhill), and does not require metabolic energy (passive). Can be measured. Permeability describes the ease with which a solute diffuses through a membrane, but depends on the characteristics of the solute and the membrane.
Factors that increase permeability: high oil/water partition coefficient of the solute increases solubility in the lipid of the membrane, decreased radius/size of the solute increases speed of diffusion, and decreased membrane thickness decreases the diffusion distance. Small hydrophobic solutes have the highest permeabilities in lipid membranes. Hydrophilic solutes must cross cell membranes through water-filled channels/pores. If the solute is an ion, its flux will depend on concentration difference and the potential difference across the membrane.
Facilitated diffusion
Characteristics: occurs down an electrochemical gradient, similar to simple diffusion. Is passive as it doesn’t require metabolic energy, but is more rapid than simple diffusion. Carrier-mediated, so it has stereospecificity, saturation, and competition. Example; glucose transport in muscle and adipose cells is facilitated by insulin. Deficiency of insulin → hyperglycemia → type I diabetes
Osmosis, Osmotic Pressure
Osmolarity: the concentration of osmotically active particles in a solution, is a colligative property and can be measured by freezing point depression. Two solutions with the same calculated osmolarity are isosmotic. If two solutions have different osmolarity, the solution with the higher osmolarity is hyperosmotic and the solution with the lower osmolarity is hyposmotic.
Osmosis: the flow of water across a semipermeable membrane from a solution with low solute concentration to a solution with high solute concentration.
Osmotic pressure: the OP increases when solute concentration increases, the higher to OP of a solution the greater the water flow into it. Water flows from hypotonic to the hypertonic solution. Colloid osmotic pressure: osmotic pressure created by proteins (plasma proteins). Reflection coefficient: a number between zero and one that describes the ease with which a solute permeates a membrane. If RC is one- the solute is impermeable and retained in the original solution, creating an OP and causing water flow. If RC is zero, the solute is completely permeable and will not exert any osmotic effect.
Cystic Fibrosis
CF is a result of a genetic defect, abnormal NaCl and water movement across wet epithelial membranes. Where these membranes line the pancreatic ductules and small respiratory airways, they produce a dense, viscous mucus that cannot be properly cleared, which can lead to pancreatic and pulmonary disorders. The genetic defect involves the glycoprotein CFTR (cystic fibrosis transmembrane conductance regulator) that normally exists in the cytoplasm of the cell and controls excretion of chloride from the cells into lumen of organs. The protein is formed in the usual manner in the endoplasmic reticulum, but does not move into the Golgi complex for processing, so it doesn’t get correctly processed or inserted into vesicles that would introduce it into the cell membrane. CF occurs when there is a mutation in the CFTR gene. The protein created by this gene is anchored to the outer membrane of cells in the sweat glands, lung, pancreas, and other affected organs. The protein acts as a channel connecting the inner part of the cell (cytoplasm) to the surrounding fluid. This channel is primarily responsible for controlling the movement of chloride from inside to outside of the cell. When the CFTR protein doesn’t work, chloride is trapped outside the cell. Lumen osmotic pressure increases and lumen will absorb water and white blood cells (formation of mucus). Can cause death, pancreatitis, hormonal issues, diarrhea, dehydration, infertility in men.
Regulation of Blood Osmolarity
When a person is dehydrated, the blood is more concentrated as the total volume is reduced. This increase in blood osmolality and OP stimulate osmoreceptors, which are neurons in the hypothalamus - via CN 9 and 10. This person becomes thirsty and, if water is available, drinks. Along with increased water intake, a person who is dehydrated excretes a lower volume of urine. The osmoreceptors stimulate a tract of axons that terminate the posterior pituitary, causing the pituitary to release antidiuretic hormone (ADH) into the blood. ADH acts on the kidneys to promote water retention, so a lower volume of more concentrated urine is excreted. Normal osmolarity in plasma is 280-303 milli-osmoles per kilogram.
Edema
Water returns from tissue fluid to blood capillaries because the protein concentration of blood plasma is higher than that of tissue fluid. Plasma proteins cannot pass from the capillaries into the tissue fluid. Therefore, plasma proteins are osmotically active. If a person has an abnormally low concentration of plasma proteins, excessive accumulation of fluid in the tissues will occur - a condition called edema will result. This may occur when a damaged liver as in cirrhosis is unable to produce sufficient amounts of albumin, the major protein in the blood plasma. Contributing factors: deficiency of proteins in blood (albumin), pregnancy, destruction of capillaries, lymphatic system disorders, cardiovascular/renal disorders, hypertension.
Hyperglycemia (BG is over 120 mg/dl) and Hypoglycemia (BG is below 50 mg/dl)
The kidneys transport a number of molecules from the blood filtrate which becomes urine.
Glucose is normally completely reabsorbed so that urine is normally free of glucose. If glucose concentration of the blood of the blood and filtrate is too high, it causes a condition called hyperglycemia. However, the transport maximum will be exceeded. In this case, glucose will be found in urine (glycosuria). This can come from the consumption of too much sugar or from inadequate action of the hormone insulin in the disease diabetes mellitus. The rate of facilitated diffusion of glucose into tissue cells depends directly on the plasma glucose concentration.
When the plasma glucose concentration is abnormally low, it’s called hypoglycemia.
Oral Rehydration
Oral rehydration therapy is effective against diarrhea because the absorption of water by osmosis across the intestine is proportional to the absorption of Na+ and the intestinal epithelium cotransports Na+ and glucose. The glucose in the mixture promotes the cotransport of Na+ and the Na+ transport promotes the osmotic movement of water from the intestine into the blood. Add small amount of salt and sugar into a glass of water
Diffusion Potential
Diffusion potential: the potential difference generated across a membrane because of a concentration difference of an ion. A diffusion potential can be generated only if the membrane is permeable to the ion. The size of the diffusion potential depends on the size of the concentration gradient. The sign of the diffusion potential depends on whether the diffusing ion is positively or negatively charged. Diffusion potentials are created by the diffusion of very few ions and therefore, do not result in changes in concentration of the diffusing ions.
Equilibrium potential for ions. Make sure to know the numbers, resting membrane is -70 mV.
Equilibrium potential: diffusion potential that exactly balances (opposes) the tendency for diffusion caused by a concentration difference. At electrochemical equilibrium, the chemical and electrical driving forces that act on an ion are equa and opposite, and more net diffusion of the ion occurs. ENa+ = +65mV, ECa2+ = +120mV, EK+ = -85mV, ECl- = -85mV. Resting membrane potential: potential difference across the cell membrane, expressed as the intracellular potential relative to the extracellular potential (-70mV). At rest, the Na+ channels are closed and Na+ conductance is low.
Action potential, Depolarization, Repolarization, and Hyperpolarization
Depolarization: makes the membrane potential less negative (the cell interior becomes less negative).
Hyperpolarization: makes the membrane potential more negative (cell interior becomes more negative). Inward current is the flow of positive charges into the cells and depolarizes the membrane potential. Outward current is the flow of positive charge out of the cell and hyperpolarizes the membrane.
Action potential: property of excitable cells (nerves/muscle) that consists of rapid depolarization, or upstroke, followed by repolarization of the membrane potential. Action potentials have stereotypical size and shape, are propagating, and are all-or-none. Occurs when the cell membrane receives a stimuli, then shows a reaction.
Threshold: the membrane potential at which the action potential is inevitable. If the inward current depolarizes the membrane to threshold, it produces an action potential. Upstroke of action potential - inward current depolarizes the membrane potential to threshold -> depolarization causes rapid opening of activation gates of the Na+ channel and the Na+ conductance of the membrane increases -> Na+ conductance becomes higher than the K+ conductance and the membrane potential is driven to (but doesn’t reach) the Na+ equilibrium potential of +65mV -> overshoot is the brief portion at the peak of the action potential when membrane potential is positive -> Tetrodotoxin (TTX) blocks these voltage-sensitive Na+ channels and abolished action potentials.
Repolarization: depolarization also closes the inactivated gates of the Na+ channel, closure of these gates results in closure of the Na+ channels and the Na+ conductance returns toward zero. Depolarization slowly opens K+ channels and increases K+ conductance to even higher levels than at rest. The combined effect of closing the Na+ channel and greater opening of K+ channels makes the K+ conductance higher than that of Na+, and the membrane potential is repolarized. Thus, repolarization is caused by an outward K+ current.
Steps: 1. Resting membrane (-70) 2. Threshold (slow opening of Na+ channel) 3. Depolarization (fast opening of Na+ channel) 4. Ep/Na = +65. 5. Repolarization (K+) 6. Hyperpolarization (-85)
Absolute and Relative Refractory Periods
Absolute refractory period: the period during which another action potential cannot be elicited, no matter how large the stimulus.
Relative refractory period: begins at the end of the absolute refractory period and continues until the membrane potential returns to the resting level. An action potential can be elicited during this period only if a larger than usual inward current is provided.
Accommodation: occurs when the cell membrane is held at a depolarized level such that the threshold potential is passed without firing an action potential - is demonstrated in hyperkalemia, in which skeletal muscle membranes are depolarized by the high serum K+ concentration. Action potentials do not occur because inactivation gates on Na+ channels are closed by depolarization, causing muscle weakness
Conduction velocity
Increased by - increased fiber size (increasing the diameter of a nerve fiber results in decreased internal resistance, thus conduction velocity down the nerve is faster) and myelination (myelin acts as an insulator around nerve axons and increases conduction velocity. Myelinated nerves exhibit saltatory conduction because action potentials can be generated only at the nodes of Ranvier, where there are gaps in the myelin sheath)
Neuromuscular junction
Neuromuscular junction: the synapse between axons of motorneurons and skeletal muscle. The neurotransmitter released from the presynaptic terminal is Ach, and the postsynaptic membrane contains a nicotinic receptor. Acetylcholine binds to Ach receptors on cell membrane → opens sodium/potassium channels → sodium causes depolarization of muscle cells → opening of calcium channel → muscle contraction
Functions: 1. Synthesis/storage of Ach in the presynaptic terminal - choline acetyltransferase catalyzes the formation of Ach from acetyl coenzyme A (CaA) and choline in the presynaptic terminal. Ach is stored in synaptic vesicles with ATP and proteoglycan for later release. 2. Depolarization of the presynaptic terminal and Ca+ uptake 3. Ca+ uptake causes release of Ach into the synaptic cleft 4. Diffusion of Ach to the postsynaptic membrane and binding of Ach to nicotinic receptors 5. End plate potential (EPP) in the postsynaptic membrane 6. Depolarization of adjacent muscle membranes to threshold 7. Degradation of Ach in synaptic cleft (Ach is degraded to acetyl CoA and choline by acetylcholinesterase - AchE)
Disease- myasthenia gravis
Caused by the presence of antibodies to the Ach receptors. Characterized by skeletal muscle weakness and fatigability resulting from a reduced number of Ach receptors in the muscle end plate. The size of the EPP is reduced, making it more difficult to depolarize the muscle membrane to threshold and produce action potentials. Treatment with AChE inhibitors - such as Neostigmine.
Neurotransmitters
Ach: secreted by Vagus nerve (CN 10), accelerates motility of GI tract. Negative effect on heart - decreases heart rate and contractility of myocardium. Deficiency → muscle weakness. Excess → muscle spasm, causes gastritis and gastric ulcer, bradycardia, decreases contractibility of myocardium
Norepinephrine: primary transmitter released from postganglionic sympathetic neurons, synthesized in nerve terminals and released into the synapse to bind with alpha/beta receptors on the postsynaptic membrane. Removed from the synapse by reuptake or is metabolized in the presynaptic terminal by monoamide oxide (MAO) and catechol-O-methyltransferase (COMT). In pheochromocytoma, urinary excretion of VMA is increased. Stimulates sweat glands, increases heart rate, increases contractibility of myocardium, relaxes smooth muscle of bronchi. Oversecretion of norepinephrine and epinephrine → hypertension, arrhythmia, excess sweating, increase blood glucose concentration, palpitation.
Epinephrine: synthesized from norepinephrine by the action of phenylethanolamine-N-methyltransferase. Is secreted, along with norepinephrine, from the adrenal medulla. Stimulates liver and increases glucose release into bloodstream - maintaining blood glucose concentration.
Dopamine: prominent in midbrain neurons, released from the hypothalamus (inhibits prolactin secretion) or from the prefrontal cortex (social behavior, decisions, problem solving) or dopamine from black substance in brain stem (fine movements of hands, head and neck, and speech) or basal ganglia (pleasure, emotional behavior). Parkinson’s involves degradation of dopaminergic neurons that use the D2 receptors (deficiency of dopamine from black substance/substantia niagra). Schizophrenia involves increased levels of D2 receptors (excess dopamine from black substance). Deficiency of dopamine from hypothalamus → infertility in women because prolactin suppresses the sex hormones (hyperprolactinemia). Excess dopamine from basal ganglia → hallucination, false pleasure feelings.
Serotonin: present in high concentrations in the brain stem, formed from tryptophan, converted to melatonin in the pineal gland. For mood, appetite, mood, sleep. Deficiency → depression and bipolar disorder.
Histamine: formed from histidine, present in neurons of the hypothalamus. Function of histamine depends on location of secretion; in lungs (vasoconstrictor/bronchoconstrictor), in skin (vasorelaxator), in stomach (increases stomach acid). Important for allergic reactions. Histamine is increased in asthmatic patients. Increased histamine in the stomach → gastritis or gastric ulcer
Glutamate: most prevalent excitatory neurotransmitter in the brain, has a kainate receptor, which is an ion channel for Na+ and K+. Involved in memory, learning, and activating sodium-potassium channels.
GABA: inhibitory neurotransmitter that is synthesized from glutamate by glutamate decarboxylase. Has two types of receptors - GABAa receptor increases Cl- conductance and is site of action of benzodiazepines and barbiturates, GABAb receptor increases K+ conductance.
Glycine: an inhibitory neurotransmitter found primarily in the spinal cord and brain stem, increases Cl- conductance.
Pheochromocytoma (excess Norepinephrine and Epinephrine secretion in adrenal medulla because of tumor)
Signs/symptoms - hypertension, excess sweating, palpitation, arrhythmia
Atom, electron, proton, neutron, atomic mass, atomic number, isotopes
Atom: smallest particle still characterizing chemical element.
Electron: have negative charge. Makes up an electron cloud surrounding the nucleus.
Proton: have positive charge, 1836 times more massive than electrons. Makes up the atomic nucleus.
Neutron: have no charge, same size as protons. Makes up the atomic nucleus.
Atomic mass: sum of protons and neutrons in an atom is equal to atomic mass.
Atomic number: number of protons in an atom.
Isotopes: have nuclei with the same number of protons as the element, but with different numbers of neutrons, therefore having a different mass number.
Chemical bonding - 3 types
Covalent bond: one or more pairs of electrons are shared by two atoms.
Ionic bond: one or more electrons from one atom are removed and attached to another atom, resulting in positive and negative ions which attract each other.
Hydrogen bond: strong attraction between a partially positive hydrogen atom and a partially negative, lone-pair-bearing atom (N, O, or F)
Acids, H+
Ionic compounds (compounds with a positive or negative charge) that break apart in water to form a hydrogen ion (H+). Strength of an acid is based on the concentration of H+ ions in the solution - the more H+, the stronger the acid. Characteristics - taste sour, react strongly with metals, dangerous and can burn your skin. Examples - vinegar, stomach acid, citrus fruits.
Bases, OH-
Ionic compounds that break apart to form negatively charged hydroxide ions (OH-) in water. The greater the concentration of OH- ions, the stronger the base. Solutions containing bases are often called alkaline. Characteristics - taste bitter, feel slippery, very dangerous and can burn your skin. Examples - sodium hydroxide, ammonia.
Neutralization reaction
When acids and bases are added to each other they react to neutralize each other if an equal number of hydrogen and hydroxide ions are present. When this reaction occurs, salt and water are formed. Example: HCl + NaOH -> NaCl + H2O.
pH
The strength of an acid or base in a solution is measured on the pH scale. The pH scale is a measure of the hydrogen ion concentration, spanning from 0 to 14 with the middle point (7) being neutral. pH greater than 7 is considered a base, while a pH less than 7 is considered an acid.
Buffer: system of molecules and ions that act to prevent changes in H+ concentration and thus stabilize the pH of a solution. In blood, pH is stabilized by the reversible reaction involving the bicarbonate ion (HCO3) and carbonic acid (H2CO3).
Blood pH: lactic acid and other organic acids are produced by the cells of the body and secreted into blood. Arterial pH remains remarkably constant at pH 7.40-0.05.
Galactosemia
Galactosemia: inherited autosomal recessive trait that affects the way the sugar galactose is broken down, due to the lack of the enzyme galactose-1-phosphate uridyl transferase. Galactose can be found in food or is the result of lactose (milk sugar) being broken down into galactose and glucose. Galactose then builds up and becomes toxic, causing the body to make some abnormal chemicals. The build up of galactose and other chemicals can cause - swollen and inflamed liver, kidney failure, ovarian failure in girls, mental growth, and cataracts in the eye. The treatment is to restrict galactose and lactose from the diet for life.
Functions of Proteins
1 Binding, transport, and storage (ex. Hemoglobin is responsible for transport of oxygen to tissue. Many drug molecules are partially bound to serum albumins in the plasma) 2. Molecular switching (conformational changes in response to pH or ligand binding can be used to control cellular processes) 3. Coordinated motion (muscle contraction is mediated by the sliding motion of actin and myosin) 4. Structural support (skin and bone are strengthened by collagen) 5. Immune protection (antibodies are responsible for reacting with specific foreign substances in the body) 6. Generation and transmission of nerve impulses (some amino acids act as neurotransmitters which transmit electric signals. Receptors for neurotransmitters are protein in nature. Ex. Acetylcholine receptor is a protein structure embedded in postsynaptic neurons) 7. Control of growth and differentiation (many hormones and growth factors that regulate cell function, such as insulin or thyroid stimulating hormone are proteins).
Marasmus - carbohydrate disorder
Marasmus: occurs when protein and caloric intake are both inadequate, characterized by energy deficiency and weak immune system
Clinical signs: dry skin, loose skin folds hanging over the gluti, axillae, etc., drastic loss of adipose tissue from normal areas of fat deposits like buttocks and thighs, afflicted areas are often fretful, irritable, and voraciously hungry, may be alternate bands of pigmented and designated hair, or flaky paint appearance of skin due to peeling. Little or no water retention is present. Potassium and sodium depletion may occur if diarrhea persists. Serum protein levels are diminished. The liver suffers acute and severe protein depletion and loss of its amino acid pool. Treatment is to first correct the electrolyte imbalance followed by a gradual feeding program.
Atherosclerosis - lipid disorder
Atherosclerosis: a condition in which fatty material collects along the walls of arteries - accumulation of lipids, cholesterol, and calcium underneath the endothelial layer of blood vessel’s wall. The fatty material thickens, hardens, and may eventually block the arteries. It occurs when fat, cholesterol, and other substances build up in the walls of arteries and form hard substances called plaque. Eventually, the plaque deposits can make the artery narrow and less flexible. This makes it harder for blood to flow. If the coronary arteries become narrow, blood flow to the heart can slow/stop, causing chest pain (stable angina), shortness of breath, heart attack, and other symptoms. Pieces of plaque can break apart and move through the bloodstream. Clots block blood flow, and if they move to the heart, lungs, or brain, can cause a stroke, heart attack, or pulmonary embolism. Normal blood calcium = 10 mg/dl
Ketone bodies, Ketoacidosis, how they accumulate, symptoms + treatment
Ketone bodies: three water soluble compounds that are produced as by-products when fatty acids are broken down for energy. They are used as a source of energy in the heart and brain, and are a vital source of fasting in the brain. Three ketone bodies are acetoacetate, beta-hydroxybutyrate, and acetone. These ketones are transported from the liver to other tissues to be reconverted into acetyl-CoA to produce energy via the Krebs cycle. Positive = fasting leads to body breaking down fatty acids, releasing ketone bodies, which are used for energy. Negative = type I diabetes (antibody destroys beta cells of pancreas, leading to deficiency of insulin) → target cells cannot uptake glucose → hyperglycemia. Overproduction of ketone bodies → decrease of pH → metabolism becomes acidic → diabetic coma
Ketoacidosis: when large amounts of ketone bodies accumulate such that the body’s pH is lowered to dangerous acidic levels. Ketone bodies accumulate easily in people with diabetes mellitus, the pancreas releases insufficient amounts of insulin/no insulin at all, so glucose goes largely undelivered. The body begins feeding on itself (breaking down muscle and fat to burn as fuel) in a desperate attempt to provide fuel - ketone bodies are the byproduct of this process. Ketone bodies are usually excreted in the urine/lungs. Excess sugar destroys blood vessles, hypertension, destroys kidney, renal failure, neuropathy (central or peripheral), electrolyte imbalance
When glucose and ketone bodies build up to very high levels, the following conditions exist: 1. Hyperglycemia: too much sugar in the blood 2. Ketoacidosis: too many ketone bodies in the blood 3. Ketonuria: accumulation of ketone bodies in the urine, when ketone is excreted sodium is excreted along with it.
Symptoms of glucose and ketone-body overload include: thirst, frequent urination, dehydration, nausea, vomiting, heavy breathing, dilation of the pupils, confusion, breath resembling the smell of fruit.
Treatment with insulin and intravenous fluids can restore normal levels of blood sugar and end Ketoacidosis and ketonuria. Can be intravenous or subdermal treatment for type 1 diabetes. Treatment for type 2 diabetes is watching diet/weight or Metformin (decreases blood sugar). Gestational diabetes happens during pregnancy, treatment is fixing diet.
Steroids are from Cholesterol
Steroids differ considerably from triglycerides or phospholipids. Cholesterol is important in the body because it serves as the precursor for the steroid hormones produced by gonads and adrenal cortex (estrogen, progesterone, androgen, testosterone, aldosterone, and cortisol)
Prostaglandins (roles)
Prostaglandin: any member of a group of lipid compounds that are derived enzymatically from fatty acids and have important functions in the animal body.
Roles: 1. Cause constriction or dilation in vascular smooth muscle cells (treat erectile disfunction) 2. Sensitize spinal neurons to pain 3. Constrict smooth muscle 4. Regulate inflammatory mediation 5. Regulate calcium movement 6. Regulate hormone regulation 7. Control cell growth
PGE2 can be harmful to temperature center and hypothalamus, causing fever
Mechanism of enzyme action, effect of temperature and pH
Enzymes are proteins that catalyze chemical reactions. Almost all processes in a biological cell need enzymes in order to occur at significant rates.
Enzyme activity can be affected by other molecules: inhibitors decrease enzyme activity (drugs/poisons), activators are molecules that increase activity, activity is affected by temperature/chemical environment/concentration of substrate, some enzymes are used commercially (synthesis of antibiotics). Enzymes use a ‘lock and key’ model. The active site is continually reshaped by interactions with the substrate as the substrate interacts with the enzyme. So, the amino acids that make up the active site are moulded into the precise positions that enable the enzyme to perform its catalytic function.
Effect of temperature and pH: an increase in temperature will increase the rate of non-enzyme-catalyzed reactions. At a few degrees above body temperature (37° C), the reaction rate reaches a plateau. A similar relationship is observed when the rate of an enzymatic reaction is measured at different pH values.
Cofactors (inorganic metal ions) and coenzymes (water soluble vitamins) - definitions
Cofactors: attachment of enzymes to cofactors allows a conformational change in the protein that allows it to combine with its substrate.
Coenzyme: participate in enzyme-catalyzed reactions by transporting hydrogen atoms and small molecules from one enzyme to another. Carries electrons and hydrogen from the cytoplasm to the mitochondria for ATP production
Oxidation and Reduction
When an atom/molecule gains electrons, it is said to become reduced. When it loses electrons, it is said to become oxidized. These are always coupled reactions. Oxygen acts as the final electron acceptor in a chain of oxidation-reduction reactions that provides energy for ATP production. These reactions involve the transfer of hydrogen atoms rather than free electrons.
Metabolism, catabolism, anabolism - definition
Metabolism: all reactions in the body that involve energy transformation are termed metabolism - these processes are the basis of life, allowing cells to grow and reproduce, maintain their structures, and respond to their environments.
Catabolism: release energy, usually by the breakdown of larger organic molecules into smaller molecules.
Anabolism: require the input of energy and include the synthesis of large energy-storage molecules, including glycogen, fat, and protein.
Aerobic anaerobic cell respiration
Aerobic respiration: requires oxygen in order to generate ATP.
Glycolysis, definition and formula
Glycolysis: metabolic pathways by which glucose - a 6 carbon sugar - is converted into two molecules of pyruvic acid, or pyruvate. At the end of the Glycolytic pathway, there is a net gain of two ATP molecules per glucose molecule.
Glucose + 2 NAD + 2 ADP + 2 P(i) -> 2 pyruvic acid + 2 NADH + 2 ATP.
NAD, FAD - function and full name
Nicotinamide adenine dinucleotide (NAD) is an important coenzyme found in cells. It acts as a carrier of electrons in the transfer of reduction potential. NAD+ is the oxidized form of NADH.
Lactic acid and Anaerobic respiration
During fermentation, pyruvate remains in the cytoplasm and is converted to waste products. In skeletal muscles, this waste product is lactic acid.
Anaerobic respiration: metabolic pathway by which glucose is converted to lactic acid.
Fermentation
Fermentation: process of energy production in a cell under anaerobic conditions - no external electron acceptor.
Ischemia
Ischemia: inadequate blood flow to an organ, such that the rate of oxygen delivery is insufficient to maintain aerobic respiration. Myocardial ischemia (inadequate blood flow to the heart) may occur from atherosclerosis, a blood clot, or by an artery spasm. Severe pain in the chest and left arm area occurs because there is increased blood levels of lactic acid, which is produced by the ischemic heart muscle.
Glycogenesis, Glycogenolysis, Gluconeogenesis - location + definition
Glycogenesis: formation of glycogen from glucose in cytoplasm of liver and skeletal muscle cells.
Glycogenolysis: conversion of glycogen to glucose 6-phosphate, occurs in the liver and skeletal muscles.
Gluconeogenesis: conversion of noncarbohydrate molecules (lactic acid, amino acids, and glycerol) through pyruvic acid to glucose, occurs in the liver.
Cori cycle location - the cycle between muscle and liver
The two-way traffic between skeletal muscles and the liver (production of lactic acid by skeletal muscles during exercise being transformed through gluconeogenesis in the liver to blood glucose) is the Corci cycle. Gluconeogenesis in the liver allows depleted skeletal muscle glycogen to be stored within 48 hours.
Structure of the Mitochondria
Mitochondria have a smooth outer membrane, surrounding a very convoluted inner membrane. The convolutions form structures called cristae. The membranes together create two compartments; the intermembrane space (compartment between the membranes) and the matrix (interior of the mitochondria)
ETC steps and Chemiosmosis steps
Steps of ETC: 1. NADH and FADH2 bring high energy electrons and protons to the cristae from either glycolysis of the Krebs cycle 2. The high energy electrons are passed to proton pumps and electron acceptors in the cristae 3. The electrons provide energy so the proton pumps can pump protons from the matrix to the outer compartment 4. As the electrons pass through the ETC, they lose energy. As they pass the last proton pump, they are low in energy and are sent back to the matrix 5. Oxygen is the final electron acceptor. When oxygen is present, it is present to water by adding protons and electrons. This removes the electrons and protons, and also provides a constant source of NAD+ and FAD. 6. The NAD+ and FAD produced in the 1st steps diffuse back to the sites of glycolysis and Krebs cycle to keep those processes going. Steps of
Chemiosmosis: NADH and FADH2 bring high energy electrons and protons from glycolysis and Krebs cycle 2. High energy electrons provide energy for the proton pumps to pump protons to the outer compartment 3. A high concentration of protons accumulate in the outer compartment. The protons thus can diffuse back to the lower concentration in the matrix 4. Passage of protons through the enzyme ATP synthase provides energy to create ATP
Uses of different energy sources
The blood contains a variety of energy sources from which to draw: glucose and ketone bodies that come from the liver, fatty acids from adipose tissue, and lactic acid + amino acids from muscles. The brain uses blood glucose as its major energy source (under fasting conditions, blood glucose is supplied by the liver through glycogenolysis and gluconeogenesis). Many organs spare glucose by using other energy sources. During severe starvation, the brain gains some ability to metabolize ketone bodies for energy.