Chemical Equilibria
1/20
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
21 Terms
reversible reaction at equilibrium
In a reversible reaction at equilibrium:
The forward and reverse reactions proceed at equal rates
The concentrations of reactants and products remain constant.
DYNAMIC EQUILIBRIUM
This is the point when the forward and backward reaction happening at the same rate, the concentration of reactants and products is constant and this can only happen in a closed system.
CLOSED SYSTEM
is one in which none of the reactants or products escape from the reaction mixture
OPEN SYSTEM
matter and energy can be lost to the surroundings
HOMOGENEOUS SYSTEM
system where all the chemicals are in the same place
LE CHATELIER’S PRINCIPLE
If a system at equilibrium is disturbed, the position of equilibrium will shift in the direction that counteracts/ lessens the effect of the change.
The position of the equilibrium refers to the relative amounts of products and reactants in an equilibrium mixture
When the position of equilibrium shifts to the left, it means the concentration of reactants increases
When the position of equilibrium shifts to the right, it means the concentration of products increases
EFFECT OF CHANGING TEMPERATURE (INCREASE)
Equilibrium shifts to favour the endothermic reaction.
As the excess heat needs to be removed from the system to counteract/ lessen the effect of the initial increase.
It will increase the yield of the endothermic products.
EFFECT OF CHANGING TEMPERATURE (DECREASE)
Equilibrium shifts to favour the exothermic reaction.
As heat needs to be gained to counteract/ lessen the effect of reduced temperature and an exothermic reaction releases heat.
It will increase the yield of the exothermic products.
EFFECT OF CHANGING CONCENTRATION (INCREASE)
Equilibrium shifts to favour the reaction that produces products/ moves right.
To get rid of the extra reaction by forming more product as more molecules are available to react.
It will increase the yield of the products.
EFFECT OF CHANGING CONCENTRATION (DECREASE)
Equilibrium shifts to favour the reaction that produces reactants/ moves left.
To counteract the effect of less reactants.
It will increase the yield/ concentration of the reactants.
EFFECT OF CHANGING PRESSURE (INCREASE)
Equilibrium shifts to favour the side with fewer moles.
This will help to release the build up in pressure and decrease the pressure.
It will increase the yield of the products on this side of the reaction.
EFFECT OF CHANGING PRESSURE (DECREASE)
Equilibrium shifts to favour the side with more moles.
Pressure has been lost.
The yield of the products on this side of the reaction will be increased.
EQUILIBRIUM REACTIONS IN INDUSTRY
A compromise is made as a low temp gives good yield but the reaction would occur at a slow rate. A high temperature would have a faster rate of reaction. A compromise is made so the temperature is high enough for a fast rate but is also low to give a good yield of the desired product.
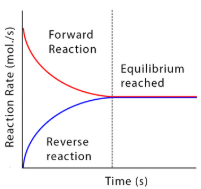
The rate of the forward reaction is high at the start of the reaction due to there being a high concentration of reactants. It decreases as the reaction progresses due to more reactants being turned into products so the rate of reaction for the reverse reaction starts to increase. The point at which the forward and backward reaction is at a constant rate is the equilibrium.
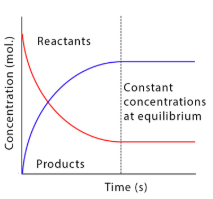
The reactants have a high concentration at the start of the reaction as none have been used up yet. There are no products at the start of the reaction. The product concentration increases as reactants are turned into products. When the concentrations are constant the system has reached equilibrium.
THE EQUILIBRIUM CONSTANT KC
The equilibrium constant is a value that expresses the relationship between the concentration of reactants and products present at equilibrium in a reversible reaction.
If Kc is large/ greater than 1 - equilibrium lies to the right as product concentration is greater than reactant concentration.
If Kc is small/ less than 1 - equilibrium lies to the left as reactant concentration is greater than product concentration.
If Kc is greater than 1010 reaction is going to completion.
Only affected by temperature
CALCULATING EQUILIBRIUM QUANTITIES/ MOLES
I - initial moles
C - change
E - equilibrium moles
Products always have an initial moles of 0 as the reaction hasn’t started yet. For products, the change is always positive and the change for reactants is negative.
Take into account the number of moles in the balanced equation and change the change in moles based by multiplying and dividing by number of moles.
A + 3B ⇌ 2C + D
I 2 1 0 0
C -0.2 -0.6 +0.4 +0.2
E 1.8 0.4 0.4 0.2
Kc UNITS

EFFECT OF CHANGING TEMPERATURE ON KC (EXOTHERMIC)
EXOTHERMIC REACTION
Increase in temp = Kc decreases as equilibrium shifts in endo direction/left/ more reactants.
Decrease in temp = Kc increases as equilibrium shifts in exo direction/right/ more products.
EFFECT OF CHANGING TEMPERATURE ON KC (ENDOTHERMIC)
ENDOTHERMIC REACTION
Increase in temp = Kc increases as equilibrium shifts in endo direction/right/more products.
Decrease in temp = Kc decreases as equilibrium shifts in exo direction/left/ more products.
EFFECT OF CHANGING CONCENTRATION OR PRESSURE AND CATALYSTS ON Kc
Kc is not affected by concentration or pressure.
This is because changing the concentration or pressure of reactants makes the equilibrium shift in the other direction to increase the concentration or pressure of the products.
Kc is not affected by catalysts.
This is because using a catalyst only speeds up the rate of reaction. They do not affect the number of particles.