Youngless: Polar vs. Nonpolar Molecules
1/21
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|

22 Terms
Polar
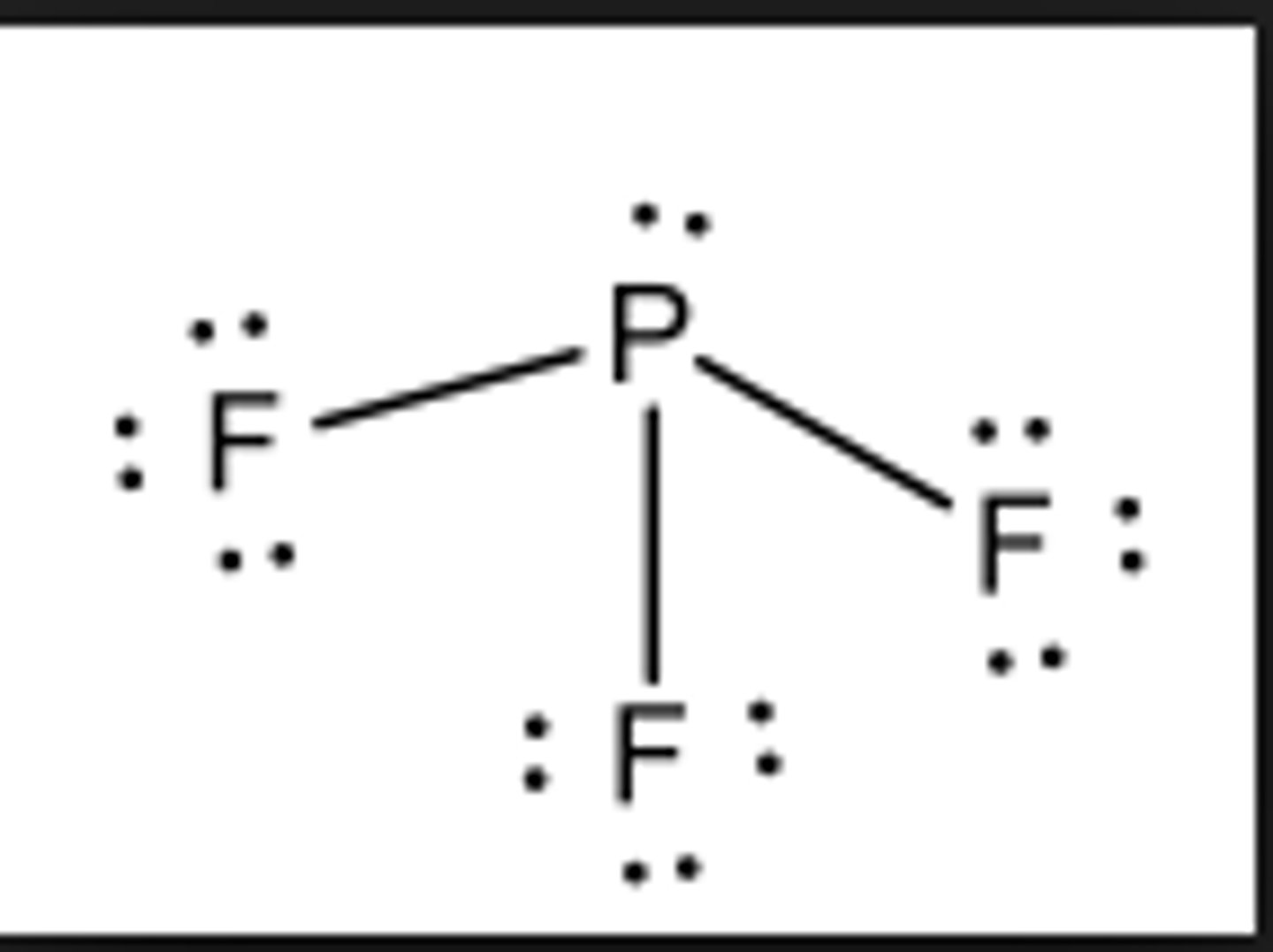
Polar or nonpolar?
Nonpolar
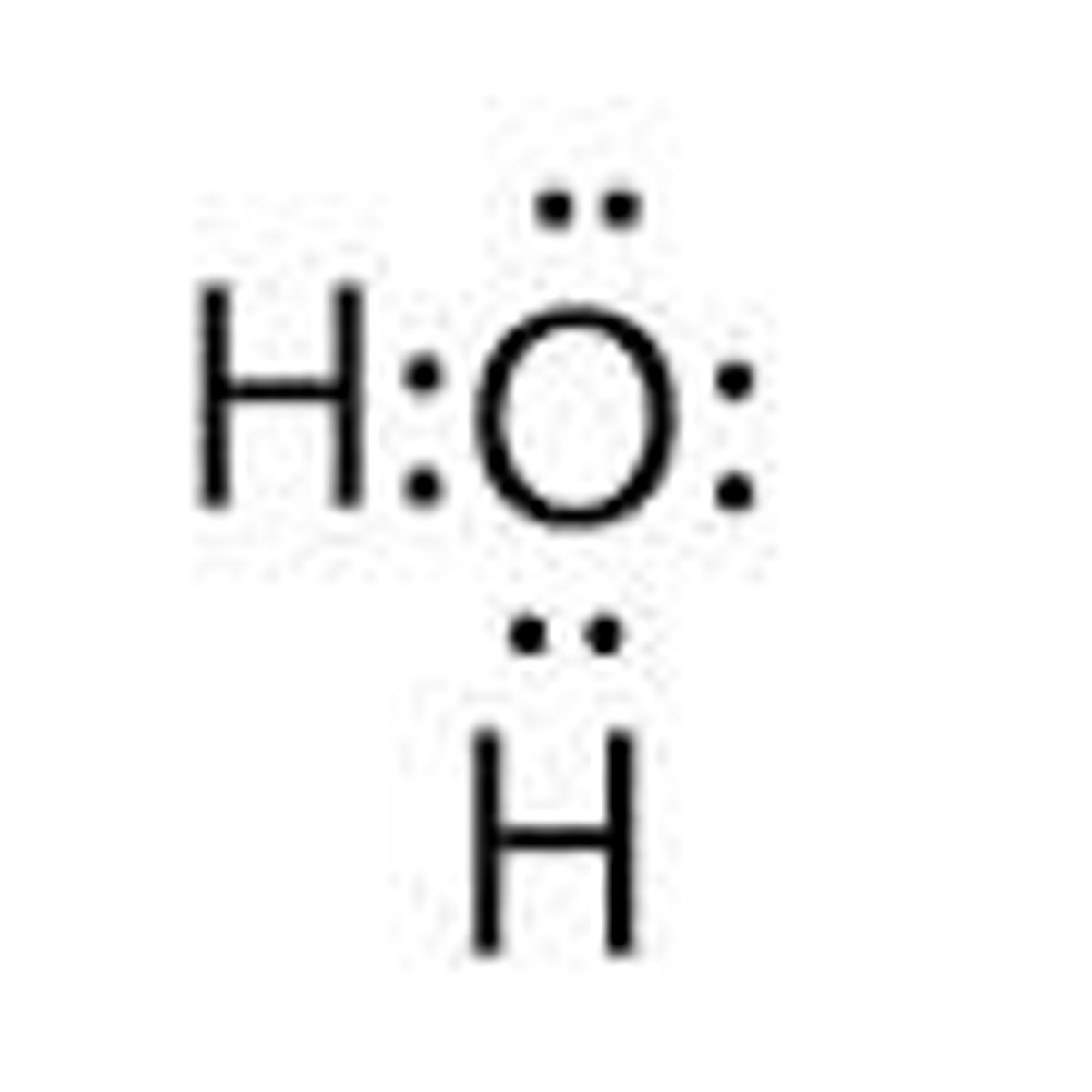
Polar or nonpolar?
Polar
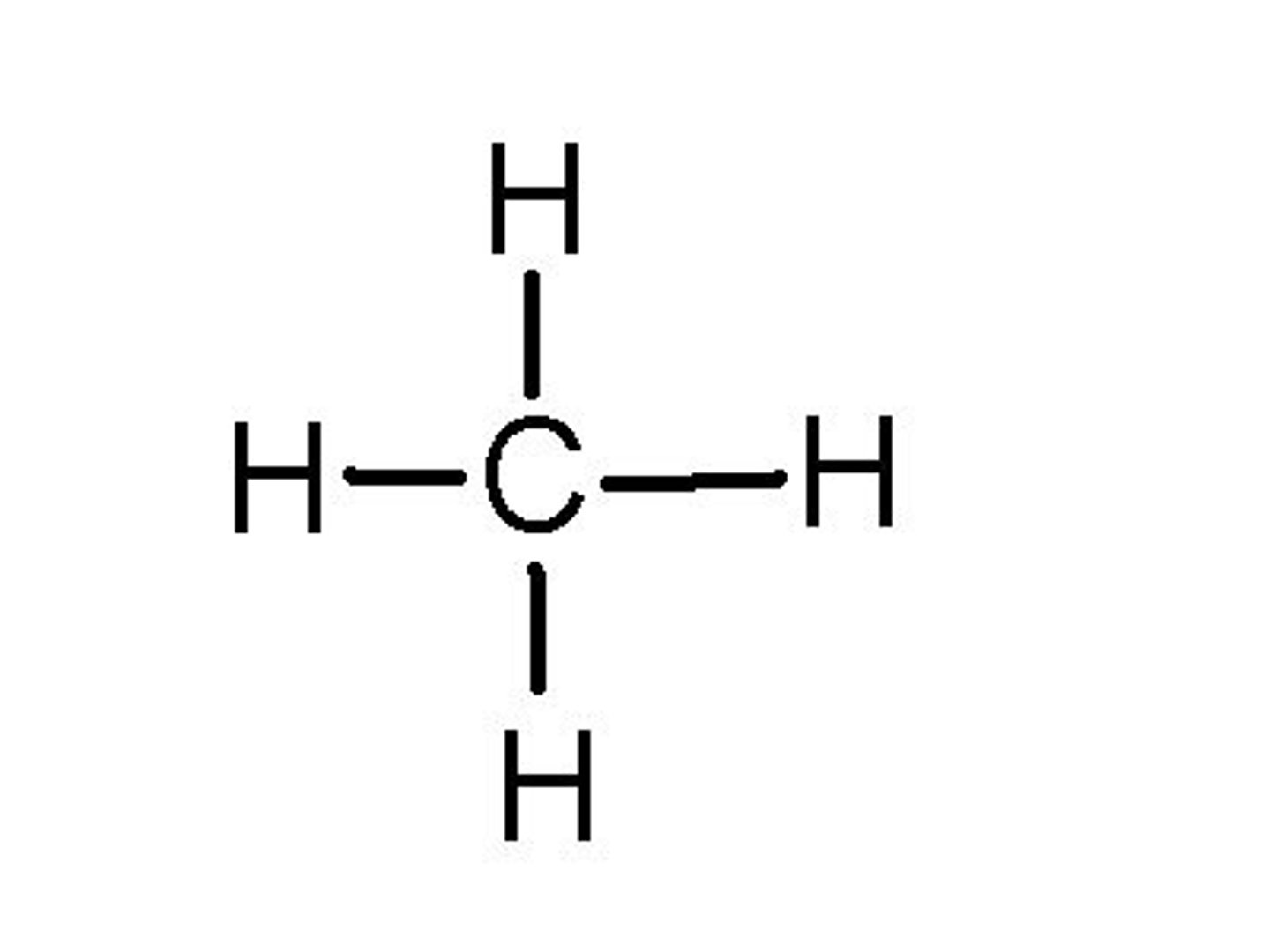
Polar or nonpolar?
Nonpolar
Polar or nonpolar?
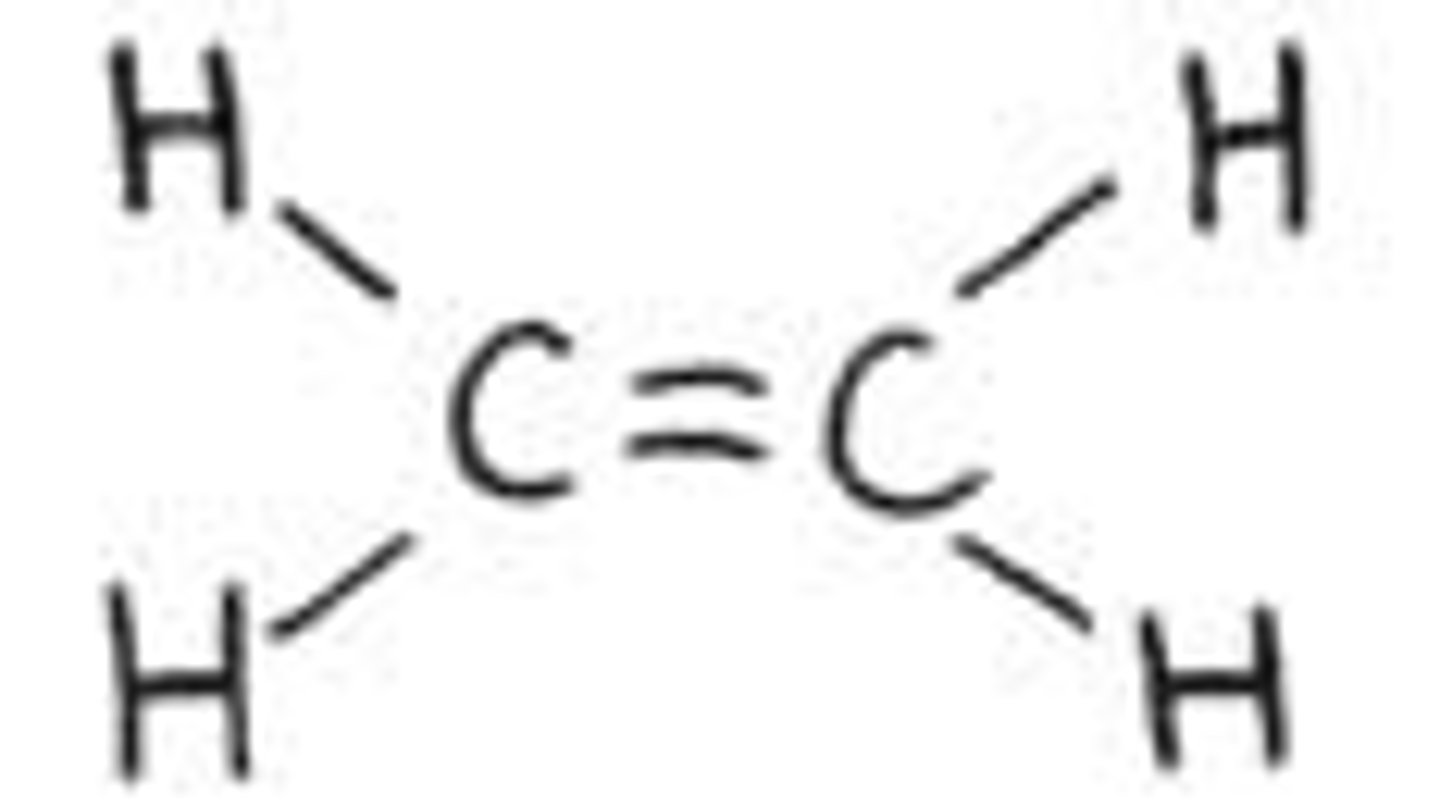
Nonpolar
Polar or nonpolar?
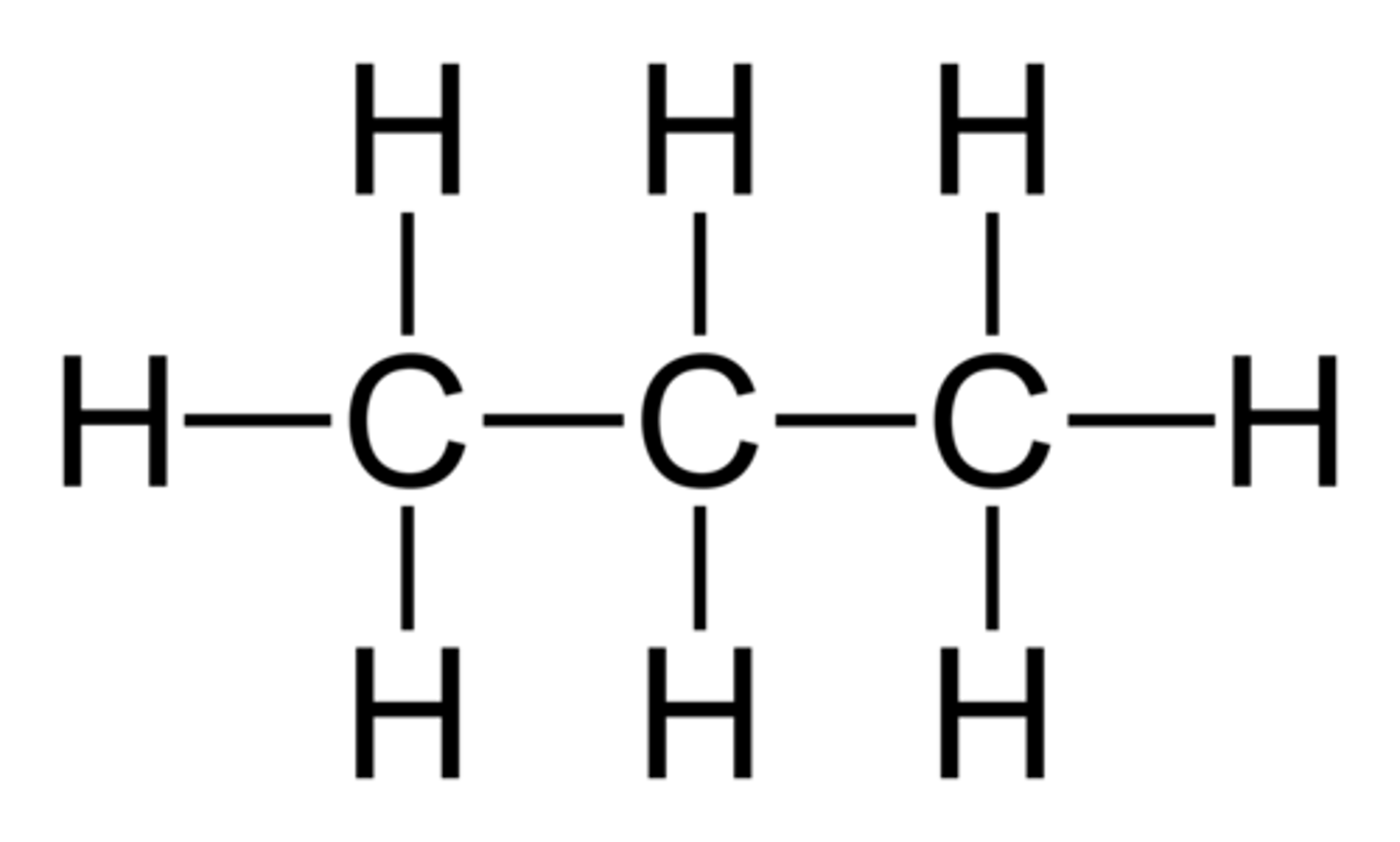
Polar
Polar or nonpolar?
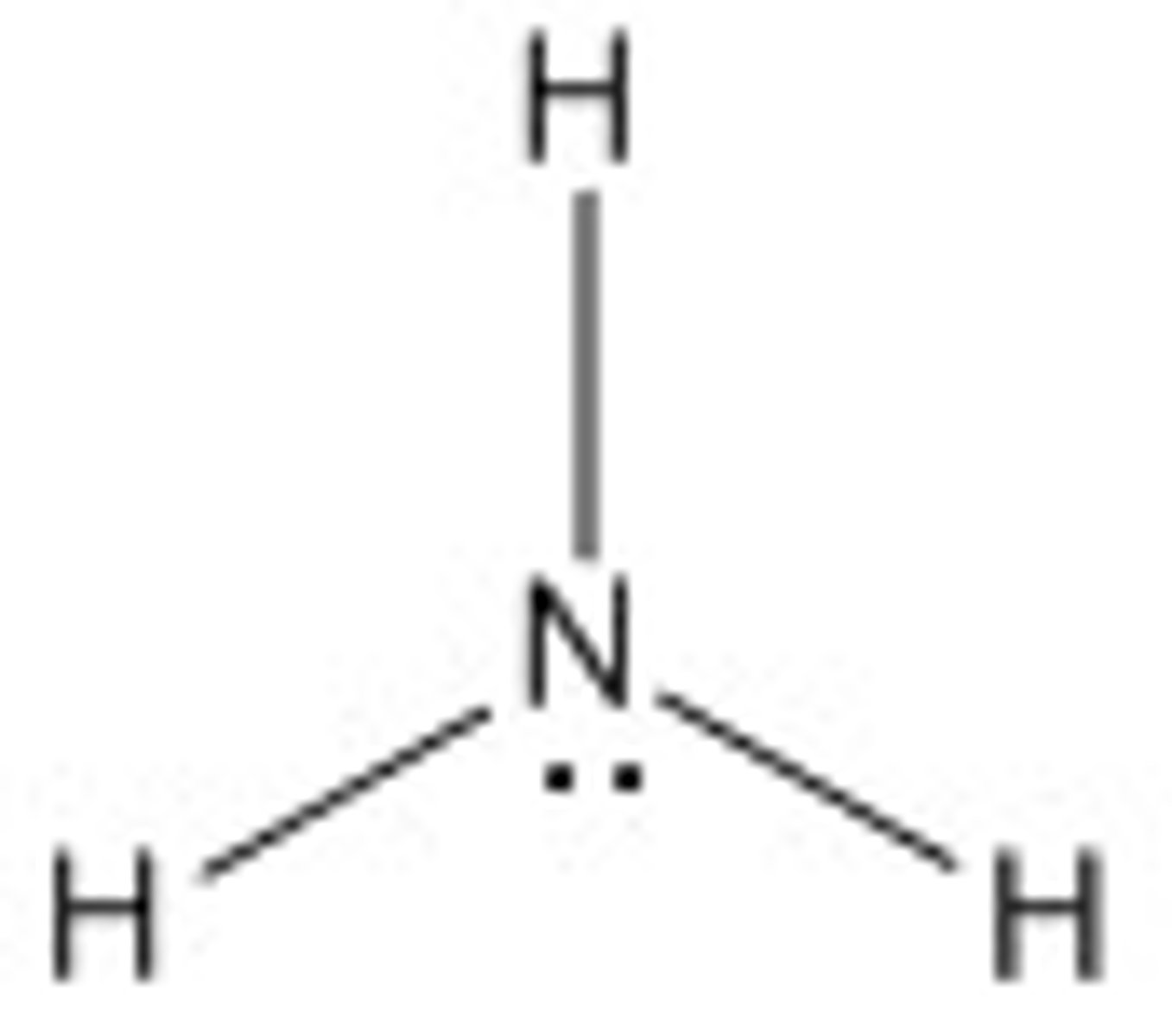
Polar
Polar or nonpolar?
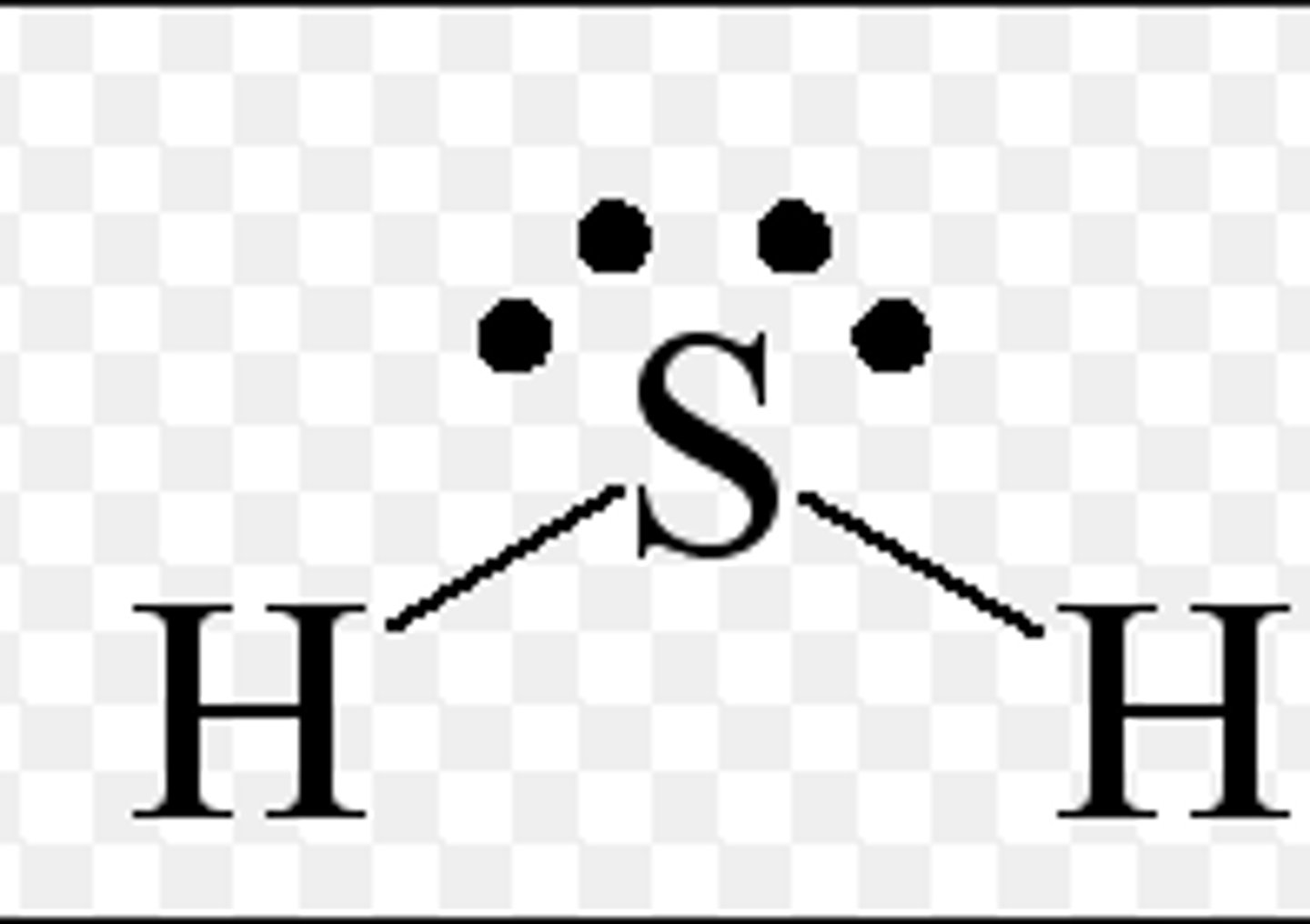
Nonpolar
Polar or nonpolar?
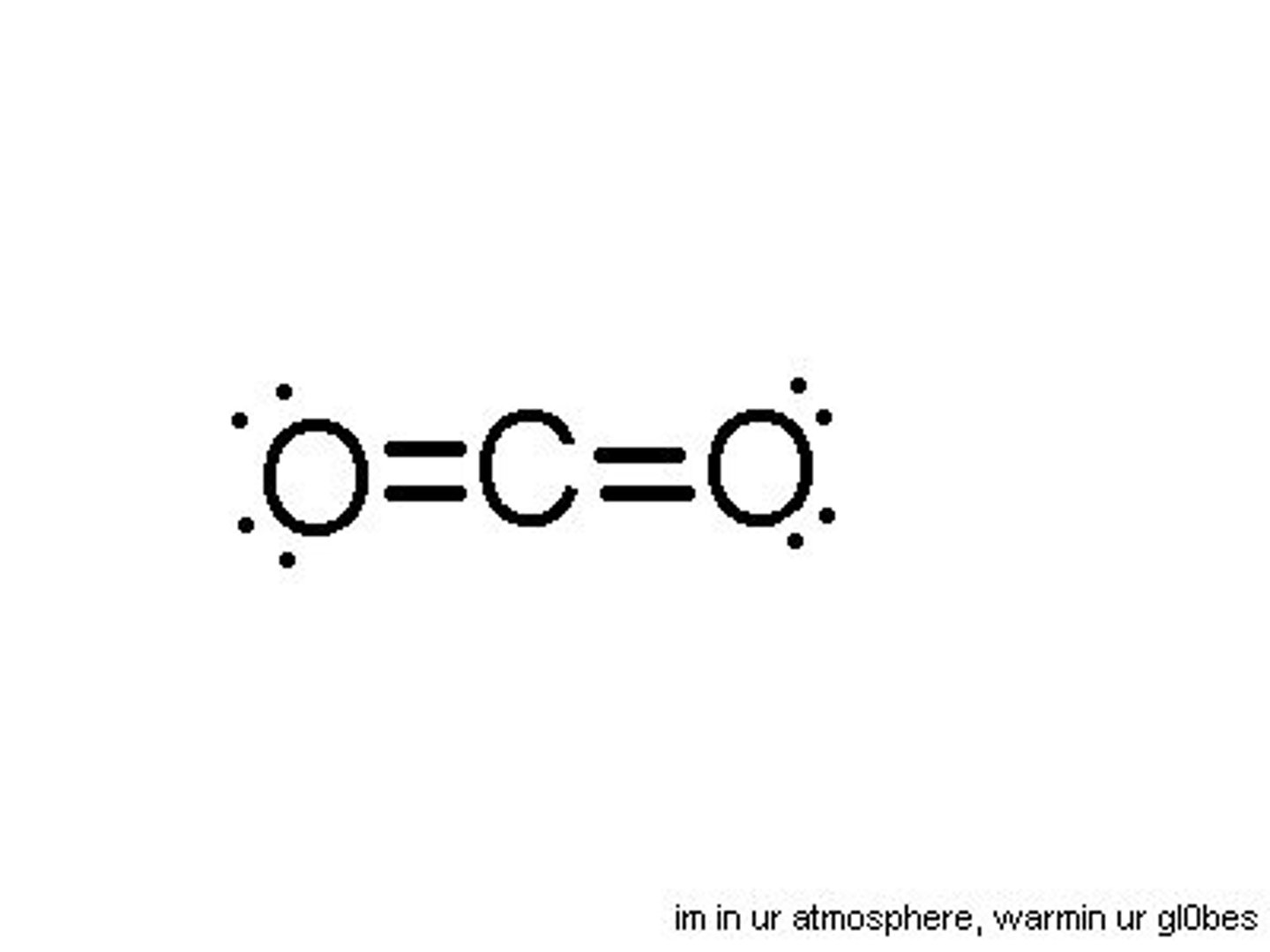
Polar
Polar or nonpolar?
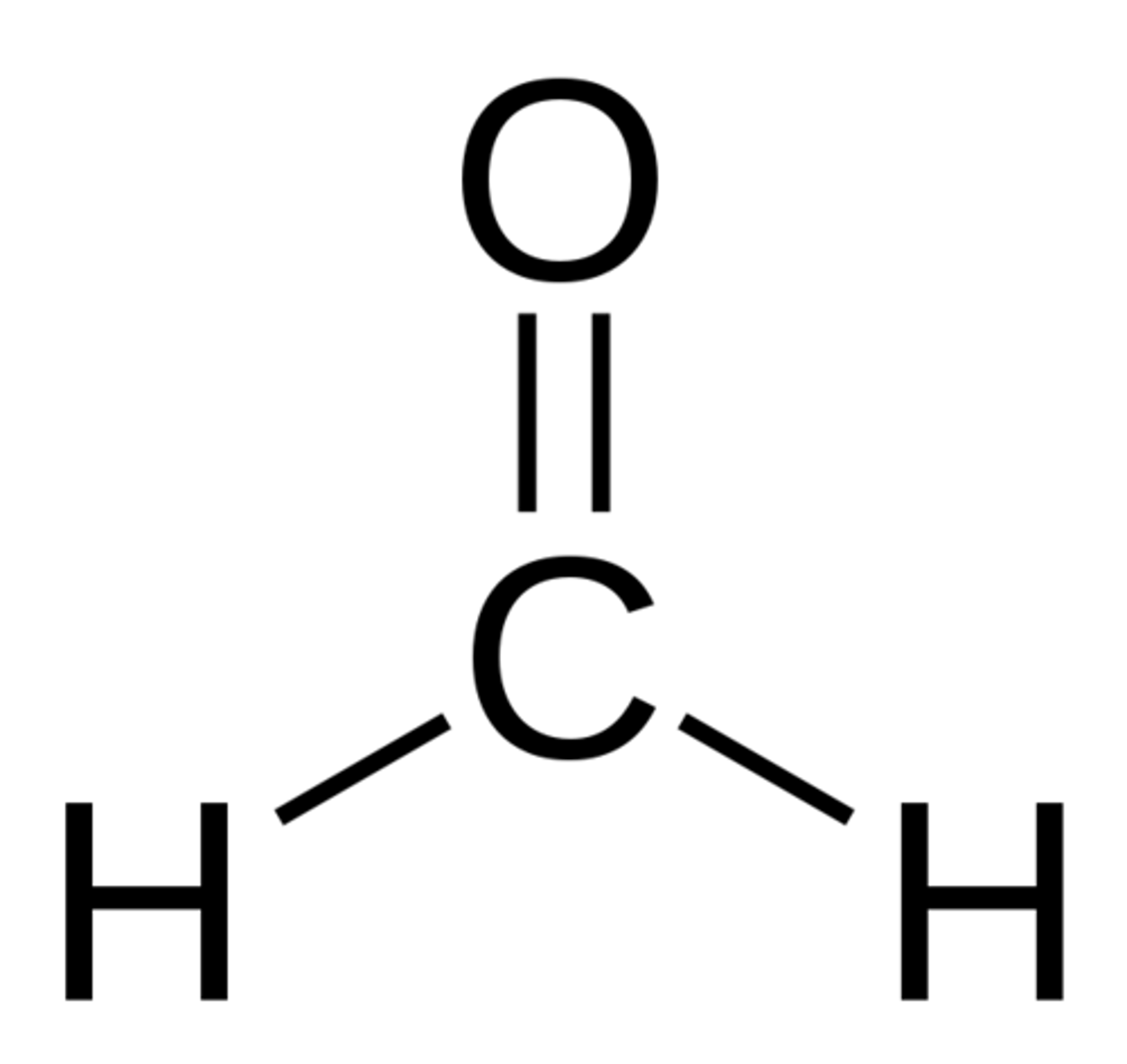
Nonpolar
Polar or nonpolar?

Nonpolar
Polar or nonpolar?
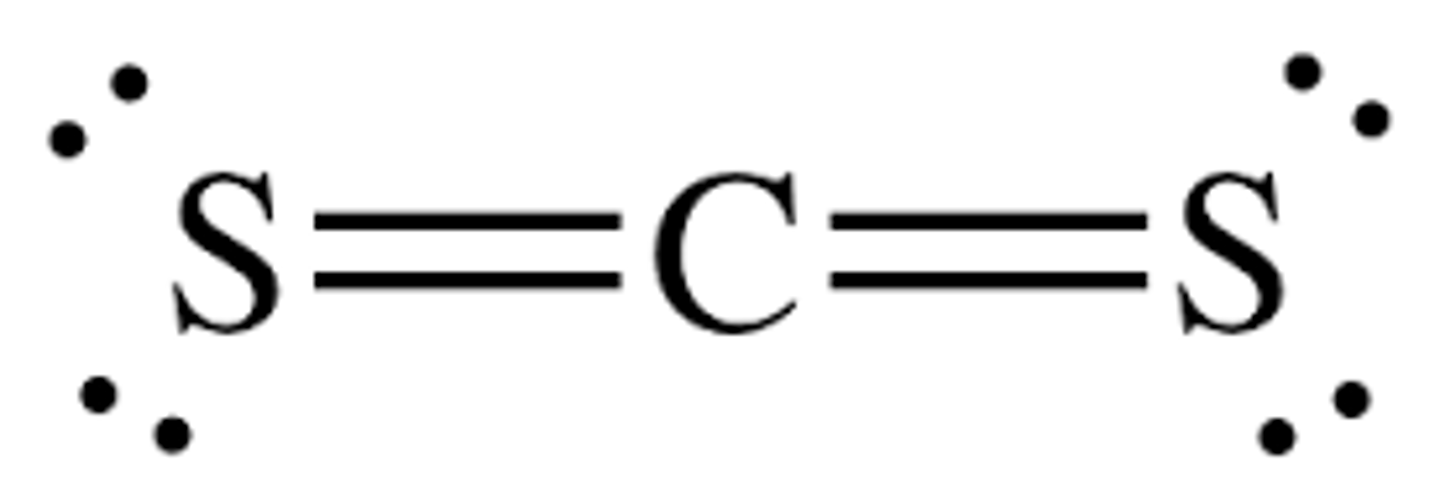
Steps to identifying polar vs nonpolar structures
1. Look at the central atom is there a lone pair of electrons on it?
yes- polar
no- go to step 2
2. Are all the side elements the same?
yes- nonpolar
no- polar
***if there are only 2 elements:
if the elements the same? nonpolar
if the elements are different? polar
polar
H₂O
nonpolar
CBr₄
polar
CBrN
nonpolar
Cl₂
nonpolar
I₂
polar
SiTe
polar definition
covalent molecules that have regions with different electronegativity values
electrons are not equally shared
has dipoles (+ and - regions)
- region; area with a higher electronegativity value (closer to F)
+ region; area with lower electronegativity value
nonpolar definition
covalent molecules that have atoms with the same electronegativity values
electrons are equally shared
In which type of bond are electrons shared equally?
nonpolar molecules
Electrons are not equally shared within this type of bond
polar