D2.3 Water potential
1/9
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
10 Terms
solvation
combination of a solvent with molecules or ions of solute
water molecules form a 3D shell around ions and charged molecules due to attraction, preventing precipitation by clumping together
movement of water molecules
particles in liquid move, do not separate until turned into gas due to intermolecular attraction
strong overall attraction between water molecules due to many bonds existing at a time despite some hydrogen bonds breaking and forming with changes in position
solute-water attractions
stronger than attraction between water molecules
restrict movement of water molecules, solutions are more viscous
osmotically active solutes
intermolecular attractions form betwen them and water (e.g. sodium, potassium, chloride ions, glucose)
water movement between 2 solutions
always movement in both directions, net movement of water from less concentrated to more concentrated
hypertonic solution - higher concentration of osmotically active solutes
hypotonic solution - lower concentration of osmotically active solutes
isotonic solutions - no difference in concentrations of osmotically active solutes, no net movement of water between two isotonic solutions
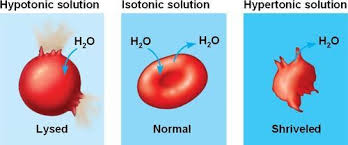
osmosis
net movement of water from less concentrated to more concentrated solution across membrane
passive movement
speed altered by changing permeability of plasma membrane
direction altered by changing concentration of osmotically active solutes inside cell
properties of plasma membrane vs cell walls
plasma membranes: phospholipids as the main constituent; thin; liquid, allowing changes of position; easily torn; semi-permeable
cell walls: cellulose as the main constituent; thicker; solid, limited changes of position; high tensile strength, very strong; freely permeable unless impregnated with waterproof material
animal cell bathed in hypotonic solution
water enters cell by osmosis, cell swells, bursts easily due to lack of support/lack of wall
effects of water movement on cells with a cell wall
turgidity - normal state of plant cell where cell becomes pressurized/swollen by entry of water/osmosis
high pressures can build up due to strong cell wall
provides support, strength under compression
wilting - caused by plant cells losing water
pressure of cytoplasm decreases
plasma membrane no longer pushes against cell wall
cell is not turgid, plant cells become flaccid
plasmolysis - plasma membrane pulls away from cell wall
when plant cells bathed in hypertonic solution, cell wall does not move (permeable to water) but volume of cytoplasm decreases
plasma membrane pulls away, eventually causing cell death
medical applications of isotonic solutions
medical procedures require isotonic solutions to prevent cell bursting or dehydrating
normal saline - isotonic NaCl solution
safely introduced to patients’ blood system via intravenous drip
used to rinse wounds and skin abrasions
used to keep areas of damaged skin moistened prior to skin grafts
basis for eye drops
frozen to consistency of slush for cooling hearts, kidneys, donor organs to be transported to hospital