Reactions at the Alpha Carbon of Carbonyl Compounds
1/30
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
31 Terms
Where is the alpha carbon located?
It is the carbon located directly next to the carbonyl carbon.
True or False: The alpha carbon is considered electrophilic.
False.
The alpha carbon is capable of holding a negative charge, and as such would not be considered electrophilic. Instead, the alpha carbon is recognized as a nucleophile.
What is an enolate?
One of the resonance forms of the conjugate base of a carbonyl that bears a negative charge at the oxygen, with a double bond between the carbonyl carbon and the alpha carbon.
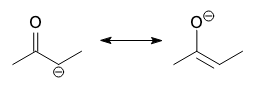
What is an enol?
The structure that is formed when an enolate ion is protonated at the oxygen, resulting in the presence of a hydroxyl group.
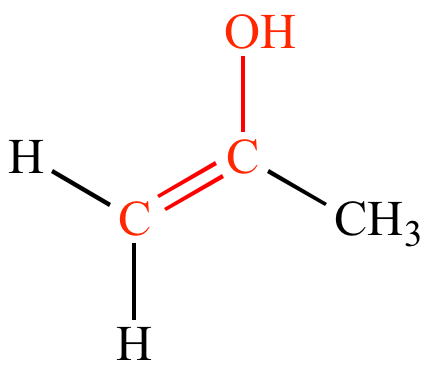
What is the difference between the keto and enol forms of a structure?
The keto form of a structure involves reprotonation at the alpha carbon, while the enol form involves reprotonation at the oxygen.
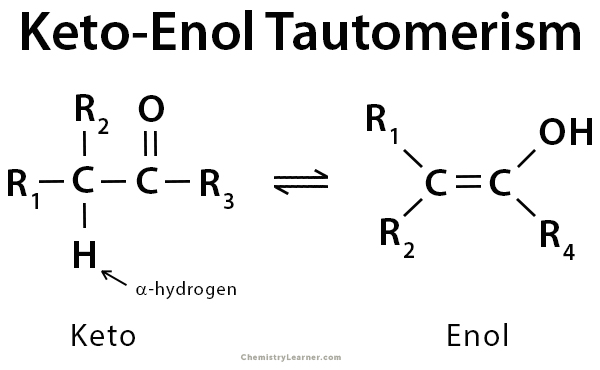
True or False: Keto and enol forms are tautomers of each other.
True.
Keto and enol forms are considered tautomers of one another, as a single proton is relocated between the two structures.
Are enols typically present in regards to simple aldehydes and ketones?
Yes, most aldehydes and ketones have a very small amount of their enol tautomers present (typically less than 1%).
True or False: Enols can only be formed under base catalysis.
False.
Enols can be formed via either acid or base catalysis.
How does deprotonation/reprotonation at the alpha carbon affect the purity of a solution?
When an enantiomerically pure substance undergoes acid or base catalysis to form either an enol or an enolate, but is then reprotonated, the proton can be delivered from either the top face or the bottom face of the structure, as neither is more preferred. Because of this, a racemic mixture consisting of two different enantiomers is formed.
What is an epimer?
A structure that varies at one stereocentre.
Under what conditions are monohalogenation at the alpha carbon possible? Specify the reactants necessary for each.
Monohalogenation at the alpha carbon can happen under base-mediated or acid-mediated conditions.
Base-mediated: A strong base, as well as a diatomic halogen (Cl2, I2, or Br2).
Acid-mediated: A strong acid, as well as a diatomic halogen (Cl2, I2, or Br2).
What is the Haloform Reaction?
A reaction in which a trihalomethyl ketone (a structure with three halogens attached to the alpha carbon) is treated with a strong base. A carboxylate salt is formed, as well as a haloform (CHCl3, CHBr3, or CHI3).
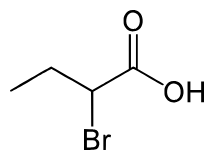
What are the reactants necessary to form this product?
1.) Br2, P
2.) H2O
Why is water added as a second step in a Hell-Volhard-Zelinsky reaction?
To hydrolyze the acid halide and leave behind a single halogen on the alpha carbon.
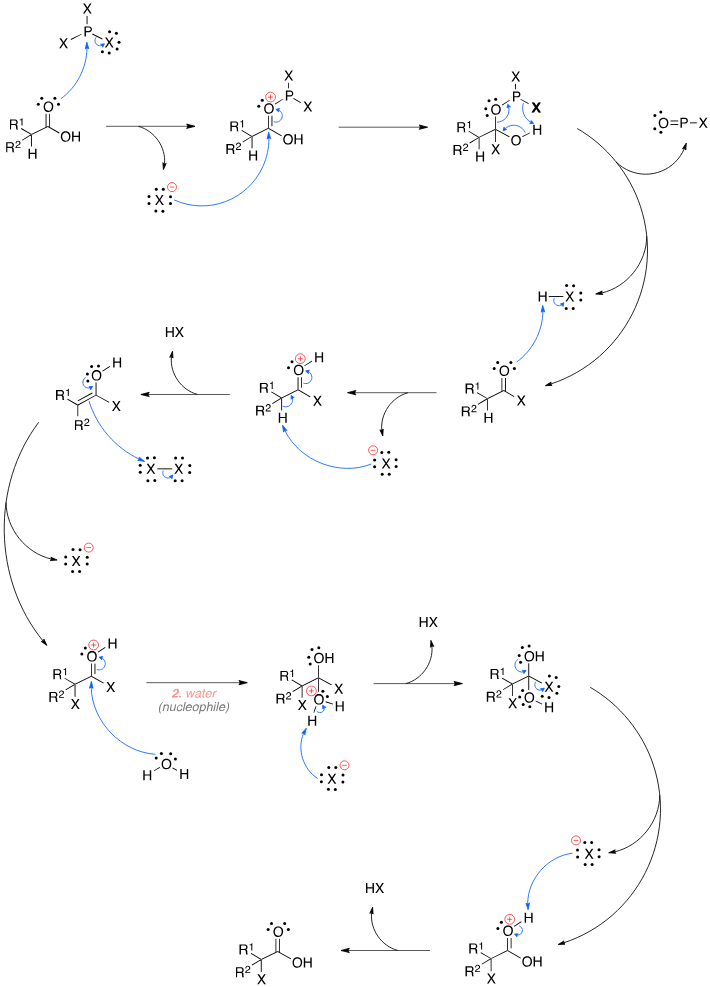
What mechanism is depicted?
The Hell-Volhard-Zelinsky reaction.
What is the preferred base to form an enolate with no equilibration? State the reasoning behind the answer.
The preferred base to form an enolate with no equilibration is the strong base LDA. This is because it is capable of fully deprotonating the alpha carbon.
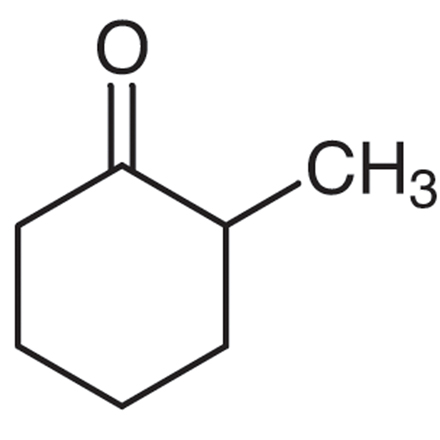
Where is the double bond of the enolate going to form following deprotonation of the alpha carbon? Explain the logic.
The double bond will form on the left-most alpha carbon. This is because LDA is a bulky base, and as such will attack the less substituted alpha carbon in order to minimize steric hinderance.
How is the thermodynamic enolate formed?
Through the use of a small, weak base (such as NaOH), which favors the more substituted enolate (regardless of steric hinderance).
True or False: Enolates tend to react with alkyl halides at the oxygen atom.
False.
When reacted with an alkyl halide, enolates tend to react at the carbon, rather than the oxygen atom.
Why is it more beneficial to use a kinetic enolate when reacting with an alkyl halide?
To ensure that no equilibration takes place, and thus only one product will be formed.
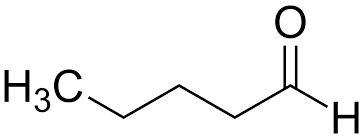
What set of reactants can be used to add a methyl group to the alpha carbon?
1.) LDA, THF, -78 C
2.) CH3Cl
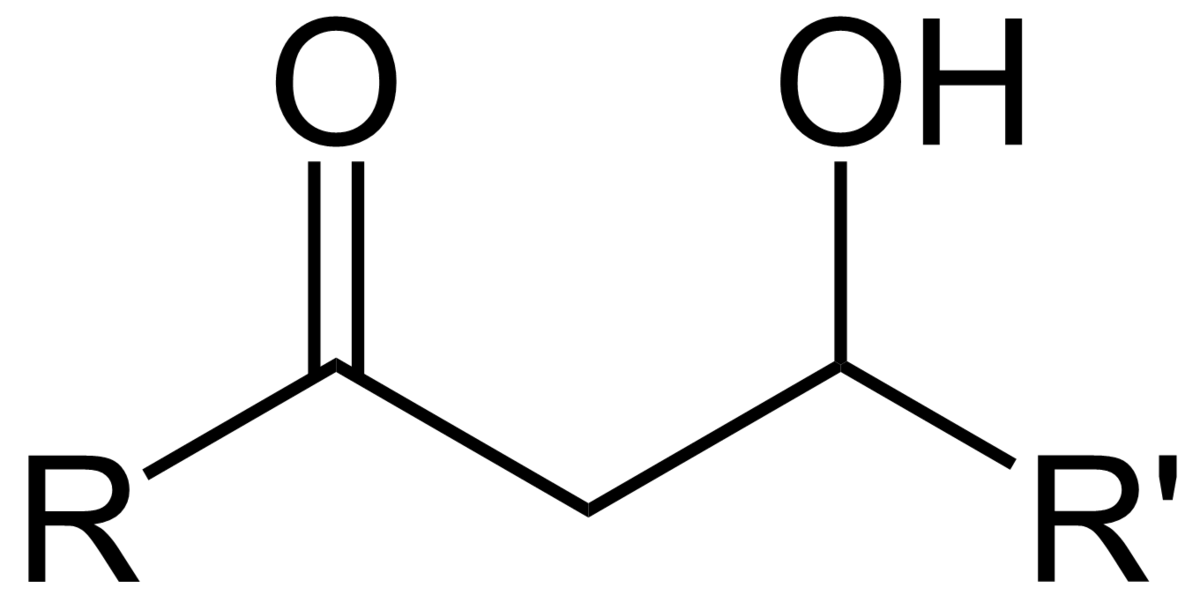
What is an aldol reaction?
A reaction in which an enolate attacks the carbonyl carbon of an aldehyde or ketone to produce a structure containing both a double bonded oxygen and a hydroxyl group.
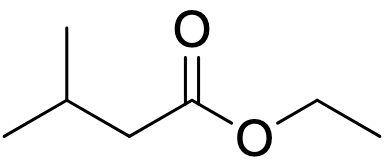
What are the reactants necessary for an aldol product to be formed from this substance? Explain the reasoning behind the use of these reactants.
1.) LDA, THF, -78 C
2.) Any aldehyde or ketone
3.) H3O+
LDA is used to preferentially form the enolate from the substance shown before the addition of an aldehyde or ketone, in order to prevent the formation of an enolate from the aldehyde or ketone. The H3O+ is added to protonate the oxygen atom.
Why are beta-dicarbonyl compounds more acidic than typical alpha carbon containing carbonyls?
Because they have greater resonance stability in their conjugate base.
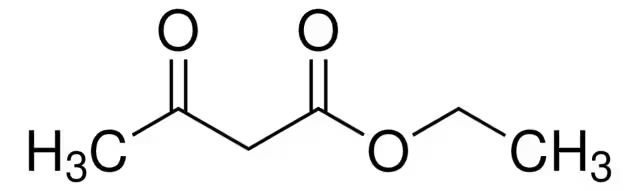
What is the name of this structure?
Ethyl acetoacetate.
True or False: A weak base can be used to monoalkylate a beta-dicarbonyl compound.
True.
A weak base can be used to monoalkylate a beta-dicarbonyl compound, as the formation of a new enolate is an incredibly slow process, making dialkylation unlikely to occur.
What is an enamine?
An amine adjacent to an alkene double bond.
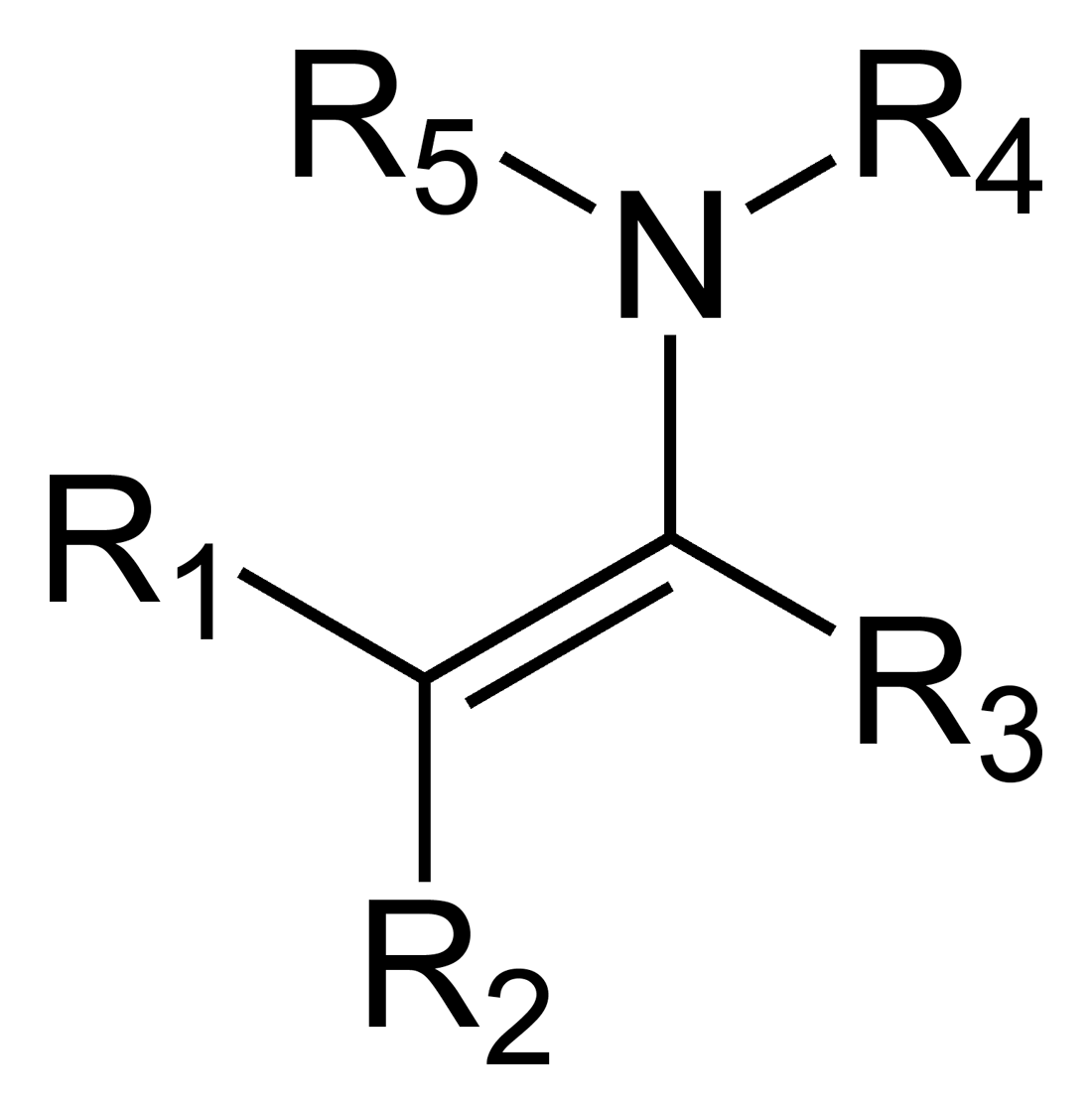
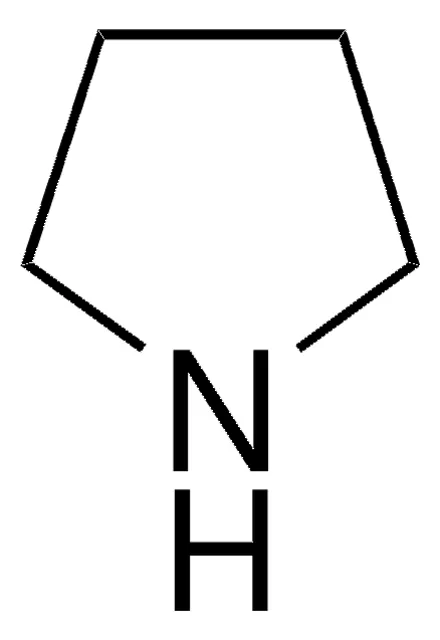
What is the name of this structure?
Pyrrolidine.
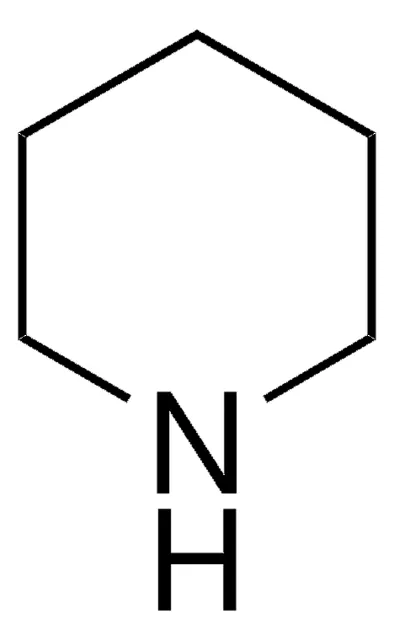
What is the name of this structure?
Piperidine.
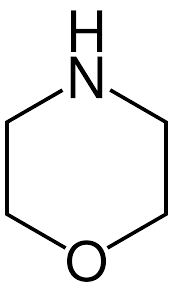
What is the name of this structure?
Morpholine.
Why are enamines used quickly after formation?
Because they are incredibly reactive with water, and prone to hydrolyzing back to the starting aldehyde or ketone.