RP1: Volumetric Solution + Acid Base Titration
1/18
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
19 Terms
What is a standard solution?
A solution of an accurately known concentration.
What is the "weighing by difference" method?
Weigh the weighing boat + solid.
Tip the solid into the beaker.
Reweigh the weighing boat (which may have traces of solid left). The exact mass transferred is: (Mass 1) - (Mass 2).
What is the purpose of rinsing the beaker, rod, and funnel into the volumetric flask?
To ensure all of the accurately weighed solid is transferred into the flask, making the final concentration as accurate as possible.
Why must a volumetric flask be inverted after making it up to the mark?
To ensure the solution is thoroughly mixed and has a uniform concentration throughout.
How do you prepare a standard/volumetric solution? (5)
Weigh by difference: Weigh the sample bottle containing the solid on a balance (ideally 2 dp or greater), transfer the solid to a beaker, and reweigh the empty sample bottle.
Dissolve: Add approximately 100 cm³ of distilled water to the beaker and stir with a glass rod until all the solid has dissolved completely.
Transfer: Pour the solution into a 250 cm³ volumetric flask via a filter funnel. Rinse the beaker and glass rod with distilled water and add these washings to the flask.
Make up: Fill the flask with distilled water until the bottom of the meniscus sits exactly on the graduation mark (use a dropping pipette for the final few drops).
Homogenise: Stopper the flask and invert it 20 times to ensure a uniform concentration.
What are the two key pieces of glassware used for accurately measuring volumes in a titration?
Pipette: Accurately transfers a fixed volume (e.g., 25.0 cm³) to the conical flask.
Burette: Accurately measures a variable volume (the titre).
How should a burette be rinsed before filling it?
Rinse it with the solution it is going to be filled with (e.g., the acid). This prevents the solution from being diluted by any residual water.
How should a pipette be rinsed before use?
Rinse it with the solution it is going to be used to measure (e.g., the alkali/standard solution). This prevents dilution.
Why is a conical flask used for the titration instead of a beaker?
Its sloped sides allow for swirling to mix the reactants without the risk of splashing the solution out.
Why is a white tile used in a titration?
To place under the conical flask, making the colour change of the indicator at the end point much easier to see.
What are concordant results in a titration?
Titres that are within 0.10 $cm^3$ of each other. (e.g., 24.50cm³ and 24.60cm³). The rough titre is usually ignored.
What is the uncertainty when reading a standard burette?
0.5cm³ for each reading. This means the total uncertainty for a titre (which requires two readings) is 1cm³.
A student calculates their percentage uncertainty to be 0.8%. They want to reduce it. Suggest one way to reduce the percentage uncertainty in this titration.
Increase the titre value (by using a more dilute solution in the burette, or a more concentrated solution in the flask).
Use a larger volume of solution in the flask (e.g., use a 50 cm³ pipette instead of 25cm³).
Use more precise apparatus (e.g., a balance with more decimal places).
(1 mark) Why is a white tile placed under the conical flask during a titration?
To make the colour change at the end point easier to see. (1)
(2 marks) A student records their titres as 25.80 cm³, 25.30 cm³, and 25.35 cm³. Calculate the mean titre the student should use in their calculation.

(3 marks) When making a 250 cm³ standard solution, explain the purpose of: (i) Rinsing the original beaker with deionised water and adding this to the volumetric flask. (1 mark)
(ii) Inverting the flask several times. (1 mark)
(iii) Using a dropping pipette to add the final few drops of water. (1 mark)
(i) To ensure all of the weighed solid is transferred. (1)
(ii) To ensure the solution is thoroughly mixed / has a uniform concentration. (1)
(iii) To ensure the bottom of the meniscus sits exactly on the graduation line / to not overshoot the mark. (1)
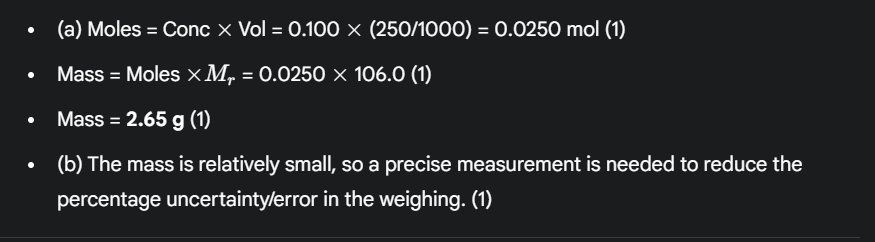
(4 marks) A student wants to make a 250 cm³ standard solution of sodium carbonate (Na₂CO₃) with a concentration of approximately 0.100 moldm⁻³. (Mr of Na₂CO₃ = 106.0) (a) Calculate the mass of Na₂CO₃ the student should weigh out. (3 marks) (b) Suggest why using a 2-decimal-place balance is important for this weighing. (1 mark)
(6 marks) Describe the method, including apparatus, a student should use to carry out a titration to find the concentration of a hydrochloric acid solution using a 250 cm³ standard solution of 0.100 $mol dm⁻³ sodium carbonate.
Burette: Rinse a burette first with deionised water, then with the hydrochloric acid solution. Fill the burette with the acid, ensuring the jet tip is full. Record the initial burette reading to 0.05 cm³
Pipette: Rinse a 25.0 cm³ pipette with the standard sodium carbonate solution.
Aliquot: Use the pipette and a pipette filler to transfer 25.0 cm³ of the 0.100 moldm⁻³ sodium carbonate solution into a conical flask.
Indicator: Add 2-3 drops of methyl orange indicator to the conical flask. Place the flask on a white tile.
Titration: Add the acid from the burette, swirling the flask, until the indicator changes colour at the end point (yellow to red/orange).
Accuracy: Repeat the titration, adding the acid dropwise near the end point. Repeat until two concordant results (titres within 0.10 cm³) are obtained.
Calculate: Record the final burette reading and calculate the titre. Use the mean of the concordant titres to calculate the concentration of the acid.