G2: Cloning and Screening of Genome Libraries
1/43
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
44 Terms
Vector
A self-replicating agent that can carry a DNA fragment into a host cell.
Commonly used vectors
Plasmids
Cosmids
Bacteriophage P1 vectors
Bacterial Artificial Chromosomes (BACs)
Yeast Artificial Chromosomes (YACs)
Plasmid
A plasmid is a small, circular, double-stranded DNA molecule found in bacterial cells. It is independent of the chromosomal DNA.
Size range of plasmids
Between 2 kb and 100 kb
Transformation
Transformation is the process by which a bacterium takes up foreign DNA (often from the environment) and incorporates it into its own genome.
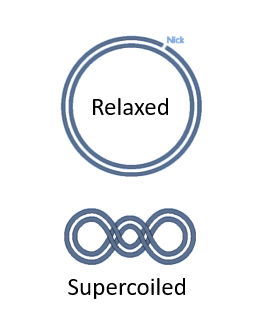
It is … to transform supercoiled plasmids in a host cell than to transform open circular plasmids.
It is easier to transform supercoiled plasmids in a host cell than to transform relaxed plasmids.
Different ways to transform plasmids into bacterial cells.
Chemical transformation: this method relies on making bacterial cell membranes more permeable to DNA by treating the bacteria with a calcium chloride (CaCl₂) solution.
Electroporation: involves using an electrical field to create temporary pores in the bacterial membrane, allowing plasmid DNA to enter the cells.
Transduction: uses bacteriophages (viruses that infect bacteria) to deliver plasmid or chromosomal DNA into bacterial cells.
Conjugation: transfer of plasmid DNA from a donor bacterium to a recipient bacterium via direct cell-to-cell contact.
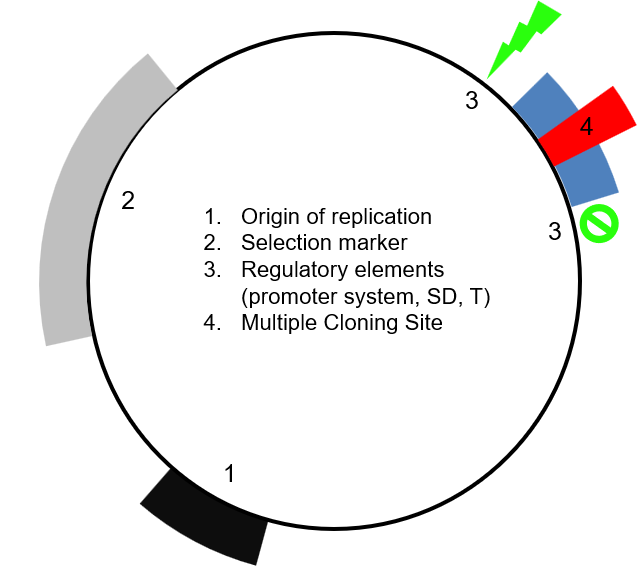
4 main elements of a plasmid vector
Origin of replication (Ori)
Selection marker
Regulatory elements
Multiple cloning site
Origin of replication
Particular sequence at which replication is initiated. It usually has a high AT content (so that separation of the two strands for replication is easier)
Three important characteristics of a plasmid that are determined by its origin of replication.
Range
Narrow range: the plasmid does not work for many bacterial species.
Broad range: the plasmid works for many bacterial species (e.g. all of the gram-negative bacteria).
Copy number
High copy number (i.e. the plasmid is present in high quantity in the host cell)
Low copy number (i.e. the plasmid is present in low quantity in the host cell)
Incompatibility
Determines if two plasmids can exist together or if one cannot exist if another plasmid is present (i.e. the plasmids interfere with each other).
Why are different plasmids with the same replication mechanism incompatible?
Different plasmids with the same replication mechanism cannot function properly together, as their replication mechanism will interfere with each other, and their copy number cannot be regulated properly.
Selection marker
Genes introduced into an organism which is required for its survival. Selection markers serve two purposes:
They allow to only keep the colonies that have successfully taken up the plasmid (if using positive selection) or to only keep the colonies that haven’t taken up the plasmid (negative selection)
They put pressure on the host organism to keep the plasmid containing the selection marker. For example, if we consider an antibiotic resistance selection marker, and put the correspond antibiotic in the media of the colonies, only the cells containing the plasmid will survive, thus pressuring the cells in keeping the plasmid.
Auxotrophic marker
An auxotrophic marker is a specific type of selection marker used in molecular biology, particularly in yeast, to select for cells that have been successfully transformed with a particular gene or plasmid. These markers exploit auxotrophy, which is the inability of an organism to synthesize a specific essential compound required for growth (e.g. essential amino acids).
How does it work?
The host organism (e.g. yeast) is engineered to have a mutation in a gene that is essential for synthetising an essential element, such as an essential amino acid. As a result, the organism becomes auxotrophic for that compound.
A plasmid vector with a functional copy of the mutated gene is transformed into cells.
The transformed cells are then placed in media that lack the essential compound, and only the cells that have successfully incorporated the plasmid will survive.
Examples of auxotrophic markers
Leu2, which encodes a compound essential for the synthesis of the leucine amino acid.
His3, which encodes a compound essential for the synthesis of the histidine amino acid.
Multiple cloning site (MCS)
A multiple cloning site (MCS), also known as a polylinker, is a short DNA sequence within a plasmid or other cloning vector that contains a series of closely spaced and unique restriction enzyme recognition sites. These sites allow to insert foreign DNA into the vector at a precise location using restriction enzymes and DNA ligase.
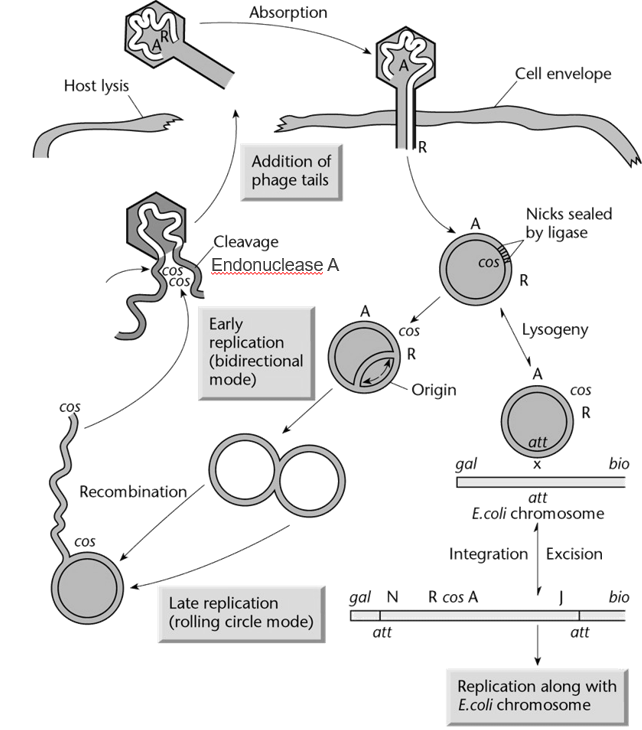
cos sites in phage DNA
cos → Cohesive end sites
Phage DNA contains two cos sites, one at each end of the linear DNA molecule.
When the phage DNA is inserted in the host bacteria, the two cos sites can recognize each other and form a circular DNA molecule. In this conformation, the DNA can be replicated and participate in either the lysogenic cycle or the lytic cycle.
The cos sites also allow enzymes involved in the assembly of phage particles to package a single DNA molecule into each phage. This process happens in the host cell (e.g. bacteria).
Capacity of phage lambda head
37 to 52 kb
Transducing particles
Particles that can transfer bacterial DNA into cells, similarly to how bacteriophage insert their own viral DNA into cells.
For example, cosmids are packaged into transducing particles.
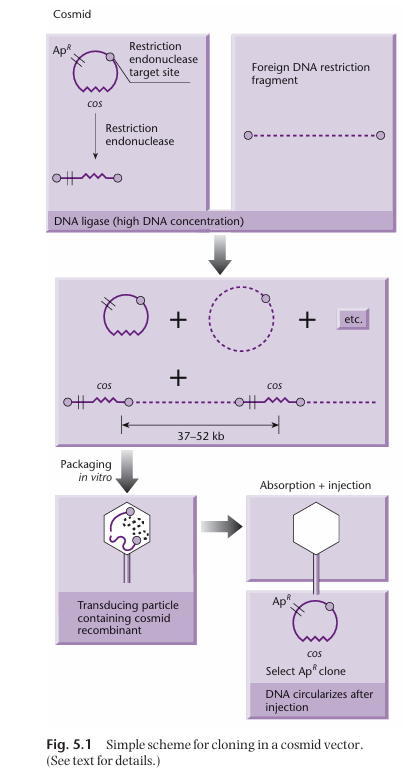
Cosmid vectors
Type of hybrid plasmid that contains a cos sequence (DNA sequence derived from bacteriophage lambda). These cos sequences allow the vector to be packaged into a transducing particle in the same way that phage DNA would be packaged in a phage.
Thus, cosmids allow foreign DNA to be transferred into cells by transduction.
How are cosmid vectors built?
DNA we want to insert into bacteria (foreign DNA) is digested to create fragments (by sonication or partial digest).
Plasmid backbone with cos site, antibiotic resistance gene, and restriction endonuclease target site is digested by said restriction endonuclease, which result in a linear fragment.
Linearised plasmid backbone and broken down foreign DNA are mixed with DNA ligase. At high DNA concentration, the foreign DNA will ligate with the plasmid backbone to create concatemers (which contains the cos site and the antibiotic gene). Note that this will also results in unwanted circular products.
The concatemers are then packaged in vitro with all the necessary protein for phage particle formation.
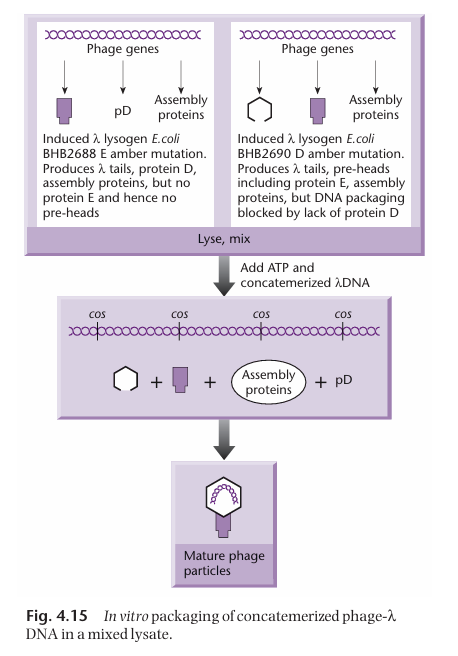
How are the proteins necessary for the formation of the transducing particles obtained?
Two different bacterial strains are necessary to get all the proteins necessary for the assembly of the transducing particle.
One of the strain has a mutated gene E (which normally encodes for the head precursor). As a result, it can make all the necessary packaging proteins except the ones for making the head. Thus, this strain cannot package DNA on its own.
The other strain has a mutated D gene. As a result, it can make all the necessary packaging proteins except the ones encoded by gene D. Thus, this strain cannot package DNA on its own.
However, when the two strains are put together, they produce all the necessary gene product for the assembly of the transducing particles, in which the cosmids can be packaged.
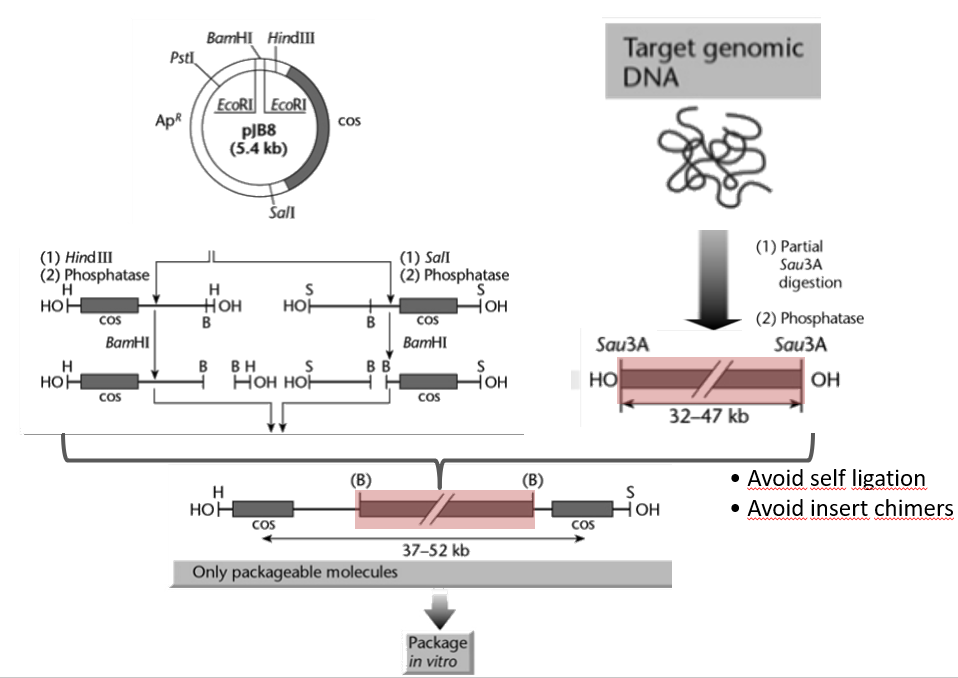
What’s a major issue we want to avoid when making genome libraries using cosmids?
We want to avoid the formation of non-contiguous fragments.
This can happen in two ways:
When generating our DNA fragments and packaging them in transducing particles, it is possible that 2 or more smaller piece of DNA (still of total number of bp between 37k and 52 k) are packaged in a single capsid. These fragments will most likely not belong to the same part of the genome, and thus once the library is built, it will not represent the genome accurately.
When ligating the cos sites with the genomic DNA fragments, it is possible that two foreign DNA fragments from different part of the genome ligate together and get flanked by cos sites. Thus, this also result in a DNA fragment that is not representative of the genome anymore.
Typical size of DNA fragments in cosmid library?
Around 45 kb
The capsid has a capacity of 37 kb to 52 kb. We also have to account for the cosmid backbone, which contains the selection marker, origin of replication, the multiple cloning site and the regulatory element. It is typically 5 kb.
45 kb + 5 kb = 50 kb
How to avoid the formation of non-contiguous fragments when building a cosmid library (i.e. chimeric DNA)?
Consider the cosmid backbone shown in the figure. It contains 3 restriction sites:
BamHI
HindIII
SalI
We carry out two different reaction with this backbone:
In the first reaction, we add HindIII and phosphatase, which results in the cosmid being cut at the HindIII restriction sites (resulting in a linear fragment), and then subsequently dephosphorylated at both ends. Thus, both end of the linear fragment have an -OH group. We then treat the DNA with BamHI, cutting two pieces.
(not done)
Bacteriophage P1 Vectors
Bacteriophage P1 vectors are cloning tools derived from the P1 bacteriophage, designed for cloning large DNA fragments (70-95 kb, almost twice as much as cosmids). They combine features of plasmids and P1 phage replication systems, making them highly efficient for maintaining large DNA inserts.
Bacterial Artificial Chromosome (BAC)
A cloning vector propagated as a mini chromosome in a bacterial host. It allows DNA insertion of large fragments (200 to 500 kb, more than double of phage P1 vector system) thanks to the use of fertility (F) factors.
As opposed to cosmids and P1 vectors, the genomic DNA fragments are ligated into the BAC in vitro (no transducing particles)
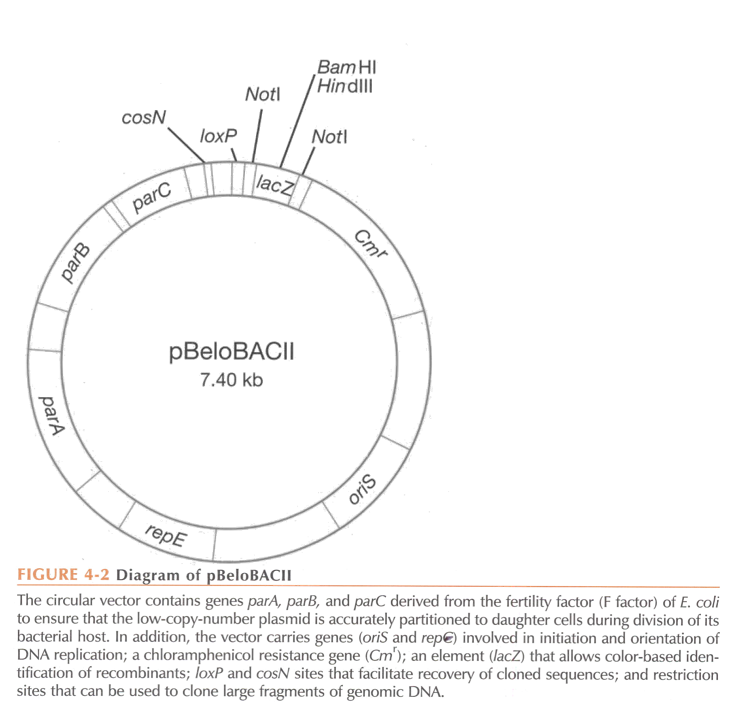
Elements of a BAC. Which elements are specific to BACs?
Origin of replication (OriS) derived from E. coli fertility plasmid (low copy vector, 1 copy/cell).
Antibiotic resistance gene chloramphenicol (Cmr)
Multiple cloning site for DNA insertion. It is within lacZ alpha or sacB (with lacZ, colonies with insert are white, with sacB, only colonies with insert will survive).
Elements specific to BACs:
Since there is only one copy per cell, partitioning of the BAC during cell replication is crucial. This is mediated by repE (encodes for helicase, enhances DNA replication) and parA, parB, and parC, which ensure correct distribution of the artificial chromosome during replication.
What process is typically used to transform BACs into host bacterial cells?
Electroporation
Electroporation
Technique used to introduce foreign DNA into cells (i.e. transformation) by applying a brief electrical pulse that temporarily disrupts the cell membrane, creating pores that allow DNA to enter the cell. The electrical pulses are very short so that the membrane opens and close quickly and stays intact.
The DNA can then be integrated into the genome or remain as plasmid DNA.
Yeast Artificial Chromosome (YAC)
A Yeast Artificial Chromosome (YAC) is a synthetic vector used for cloning large DNA fragments in yeast cells. It is particularly useful for studying large eukaryotic genomes due to its ability to carry DNA fragments up to 2 megabase (Mb) in size. It is a self replicating element.
How are YACs transformed into yeast cells?
Chemical transformation. Important elements of the process include:
Lithium acetate ensures the permeabilization of the cell membrane.
Heat shock: combination of lithium acetate with heat allows the plasmids to enter the cell.
Polyethylene glycol is a viscous substance that ensures that the aqueous phase containing the transformation components (plasmids) are kept close to the cell membrane → crowding agent.
Single-stranded DNA needs to be added in addition to the plasmid DNA. This is because the cell surface is positively charged, which means that the negatively charged plasmids will stuck to the outside of the cell rather than going inside. The ssDNA binds more strongly than dsDNA to the wall surface, ensuring that the double-stranded plasmids do not get stuck on the cell wall and can entre the cell.
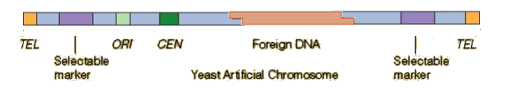
Key elements of YACs
Autonomously Replicating Sequence (ARS):
A yeast origin of replication (ORI on the schematic).
Ensures that the YAC replicates autonomously within the yeast cell.
Centromeric Sequence (CEN):
Enables proper segregation of the YAC during cell division by mimicking yeast chromosome behaviour.
Telomeric Sequences (TEL):
Protect the ends of the YAC and prevent degradation, mimicking natural chromosome ends.
Selectable Markers:
Allow selection of yeast cells containing the YAC.
Common markers include genes conferring the ability to grow in specific nutrient-deficient media, i.e. auxotrophic markers (e.g., URA3, TRP1, LEU2).
Multiple Cloning Site (MCS):
Contains restriction enzyme recognition sites for the insertion of large DNA fragments.
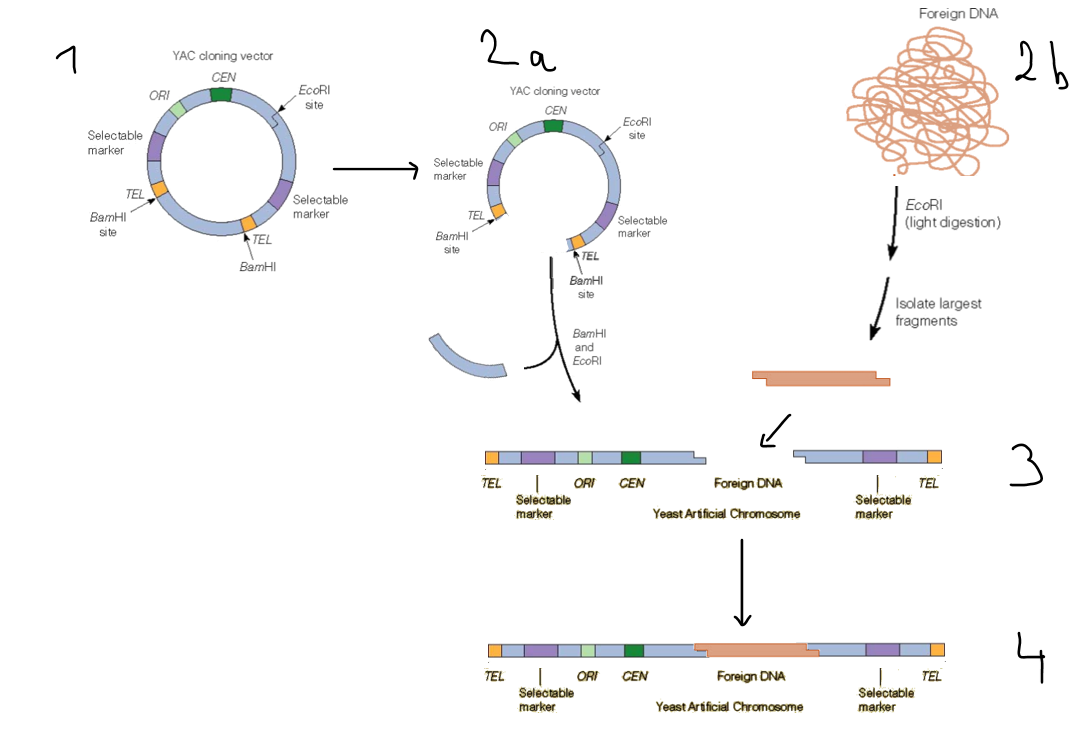
How are YACs constructed
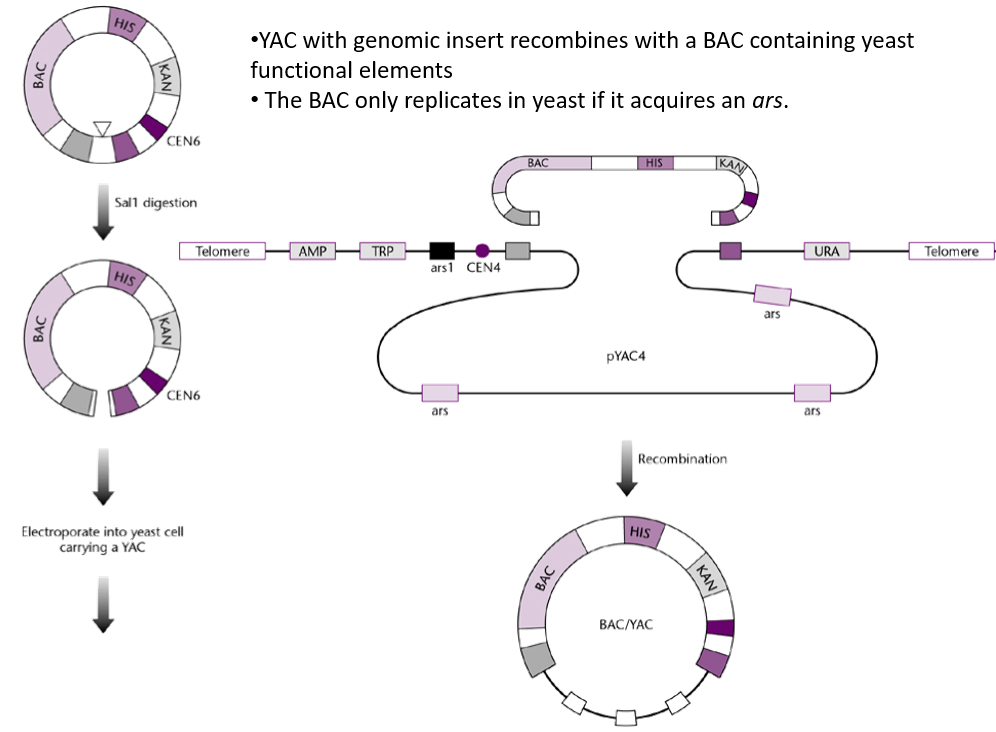
What are the problems associated with YACs, and how is this solved?
Instability:
Large DNA inserts can sometimes rearrange (homologous recombination) or be lost during yeast cell replication.
Chimeric Clones:
Incorrect assembly of inserts may lead to hybrid clones containing unrelated DNA fragments.
Not easily separated from host DNA
Pulsed-field electrophoresis required to separate host DNA from YAC DNA.
Solution is to circularise the YAC so it is more easily identifiable.
These issues can be mitigated by circularising the YACs with a BAC → BAC/YAC.
The BAC/YAC is circular, meaning that it is more easily separated from the linear host DNA. Additionally, it allows to stably maintain the DNA insert.
Differences between cosmids, P1 vectors, BACs, and YACs.

4 main steps for the construction of DNA library.
DNA fragmentation
Ligation into vectors
Introduction into host cells
Selection or screening
What are two different ways to fragment DNA to build a genomic library?
Restriction endonuclease digestion (e.g. partial digestion)
Mechanical shearing
Explain what restriction endonuclease digestion is and how it is used for building genomic libraries.
Restriction endonuclease digestion is a molecular biology technique in which a specific restriction endonuclease enzymes are used to cut DNA at particular sequences known as recognition sites.
This technique is used to fragment DNA for building genomic libraries. However, using restriction endonucleases and restriction sites means that the fragmentation of DNA is not random, as it will always be cut at the restriction sites of the restriction endonuclease(s) being used. When building genomic libraries, we want the fragmented DNA to be random, of various lengths, and overlapping to an extent.
This is partially solved by using partial digest, which is a technique used to generate a range of DNA fragments by exposing DNA to a restriction enzyme under conditions that do not allow it to cut at all of its recognition sites. This results in the DNA being cleaved at some, but not all, of the sites, producing fragments of various lengths.
→ Partial digest results in semi-random DNA fragmentation (not fully random because the enzymes will still only cut at the restriction sites.
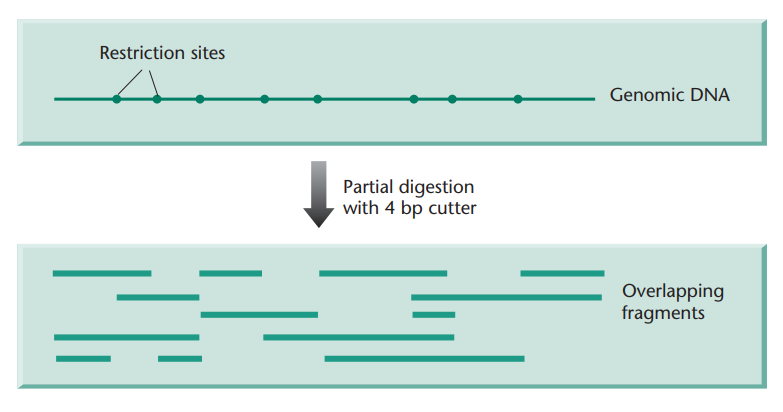
In addition to partial digest, what can be done to increase the randomness of the DNA fragments?
By using more than one restriction endonuclease.
Explain what mechanical shearing is and how it is used for building genomic libraries.
Mechanical shearing is a method used to fragment DNA into smaller pieces by applying physical force. This technique does not rely on enzymes like restriction endonucleases and instead breaks DNA randomly, making it a useful tool for generating random DNA fragments for building genomic libraries.
This is done using a sonicator, which vibrates at a high frequencies, resulting in the breakage of the DNA molecules.
What is one downside of mechanical shearing and how is it addressed?
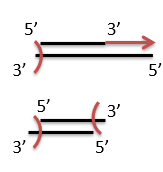
Mechanical shearing using a sonicator results in DNA fragments with “uneven” ends, unlike the use of restriction endonuclease, which results in all DNA fragments having sticky ends corresponding to the endonuclease used.
This is solved by using DNA polymerase to extend the 3’ ends and exonucleases to cut overhanging 3’ ends, which results in blunt end DNA fragments. This process is called polishing.
When using sonication, what does the average size of the DNA fragments depend on?
Frequency of the sonicator
How long the DNA is being sonicated
What does the size of a genomic library (i.e. the number of clones) depend on?
The size of the genome
The size of the fragments
Carbon & Clarke formula
Answers the question: How many random clones are needed to ensure the presence of a sequence in a library?
P = 1 - (1 - f)N
P is the probability of finding a specific sequence in the library.
N is the number of recombinants
f is the fraction of the genome per insert (f=i/G)
From this, we get:
N = ln(1 - P) / ln(1 - f)
By setting P to 0.99, we can find the number of clones to screen to get the sequence of interest with a probability of 0.99.