Solvents and Substrates
1/33
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
34 Terms
HX

HX
ROOR
H3O+ (H2SO4, H2O)
Hg(OAc)2, H2O
NaBH4

BH3 x THF
NaOH, H2O2
Br2
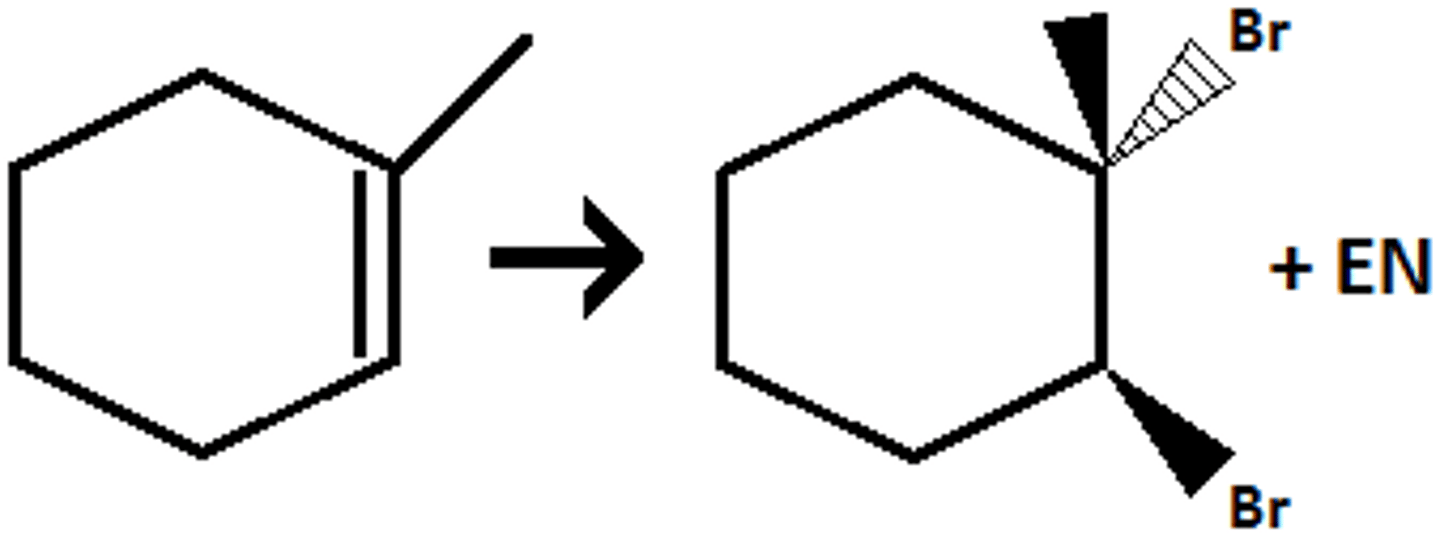
Br2
H2O
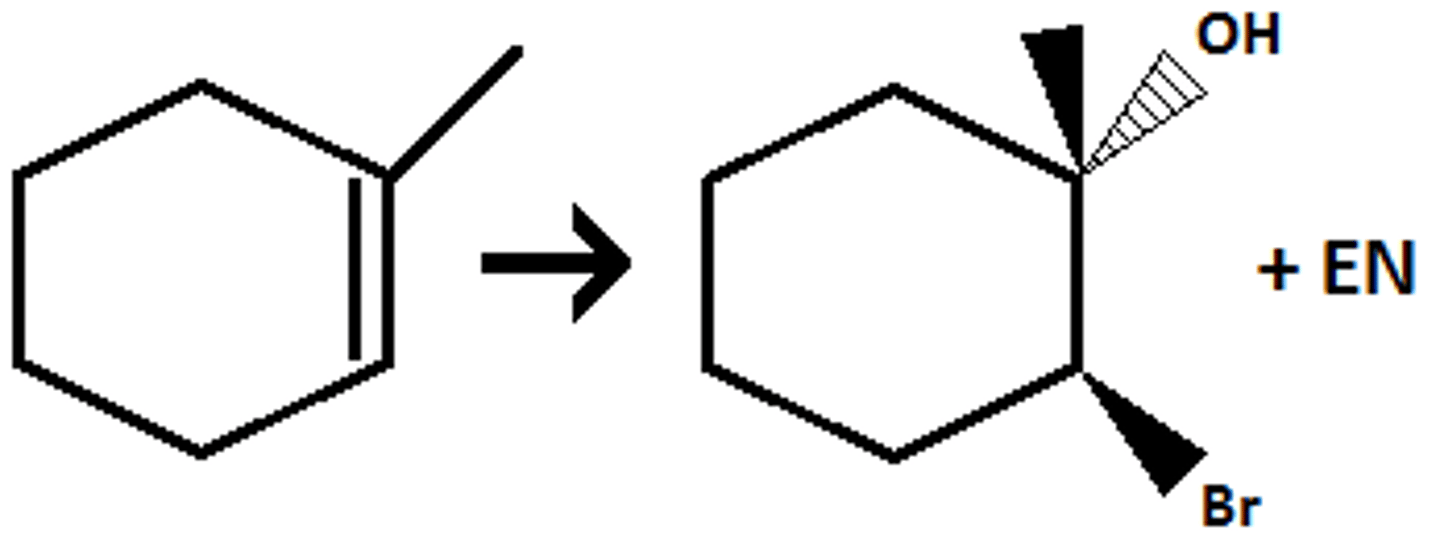
mCPBA
H3O+
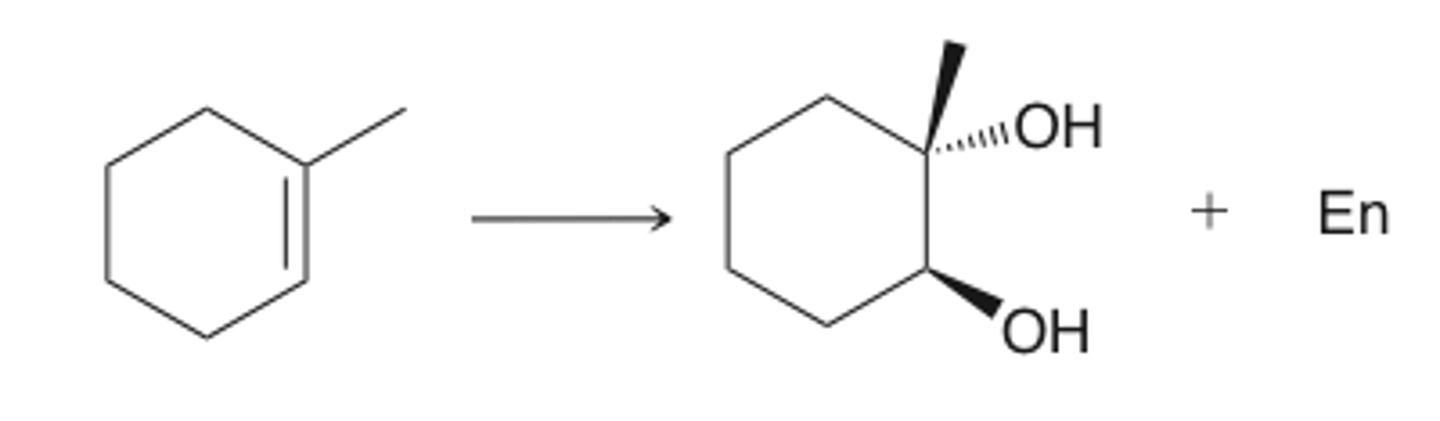
OsO4
NMO
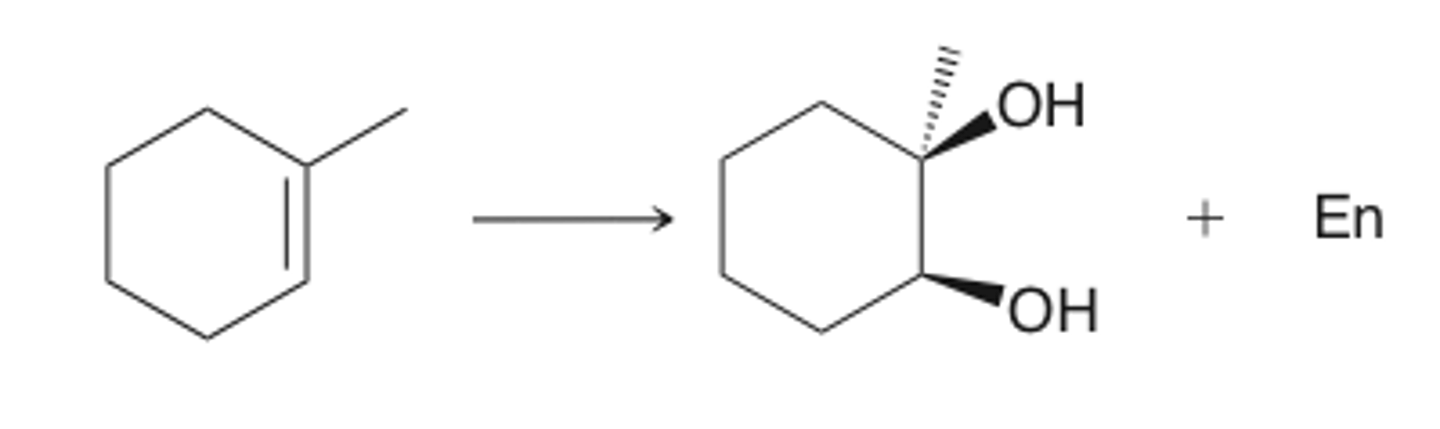
O3
DMS
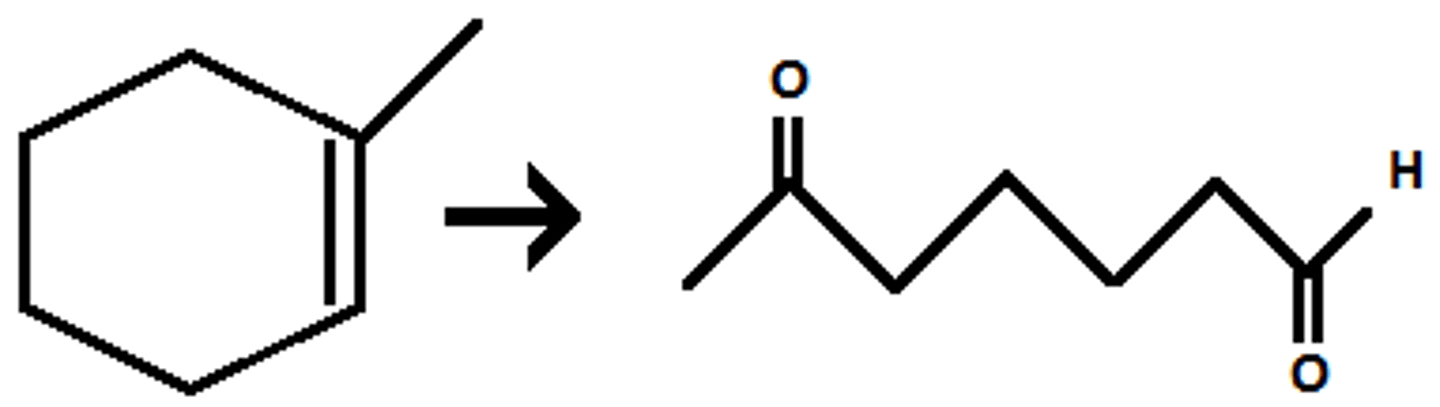
H2
Lindlar's catalyst
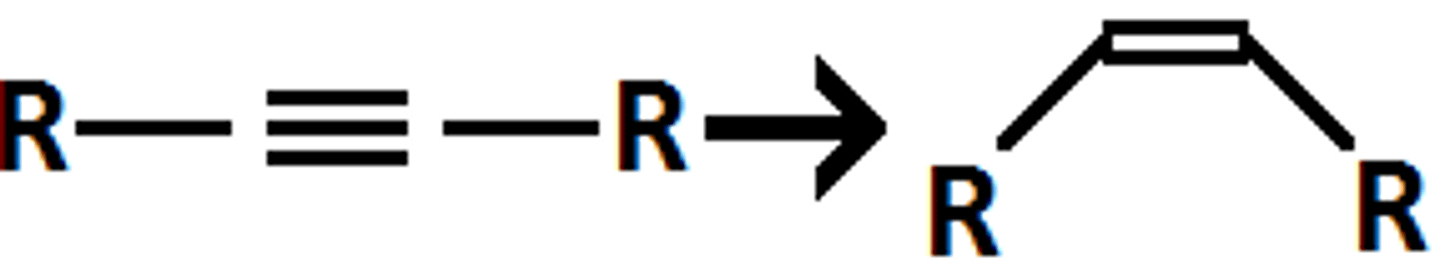
H2
Pt
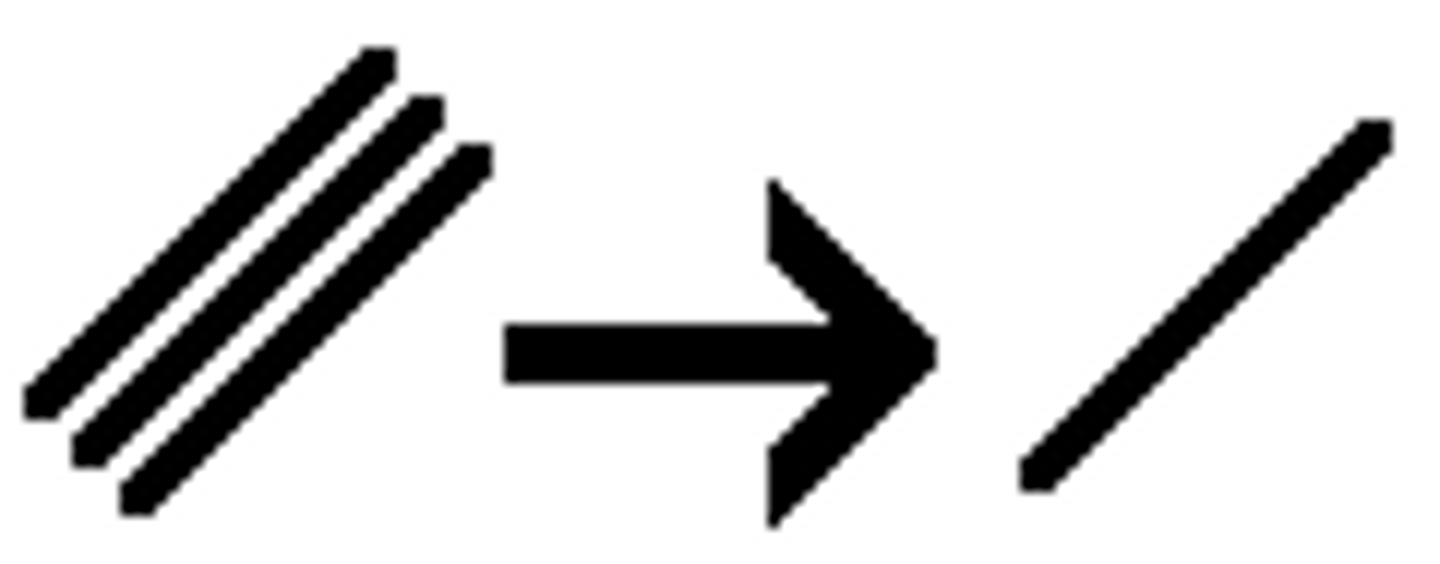
Na
NH3 (l)
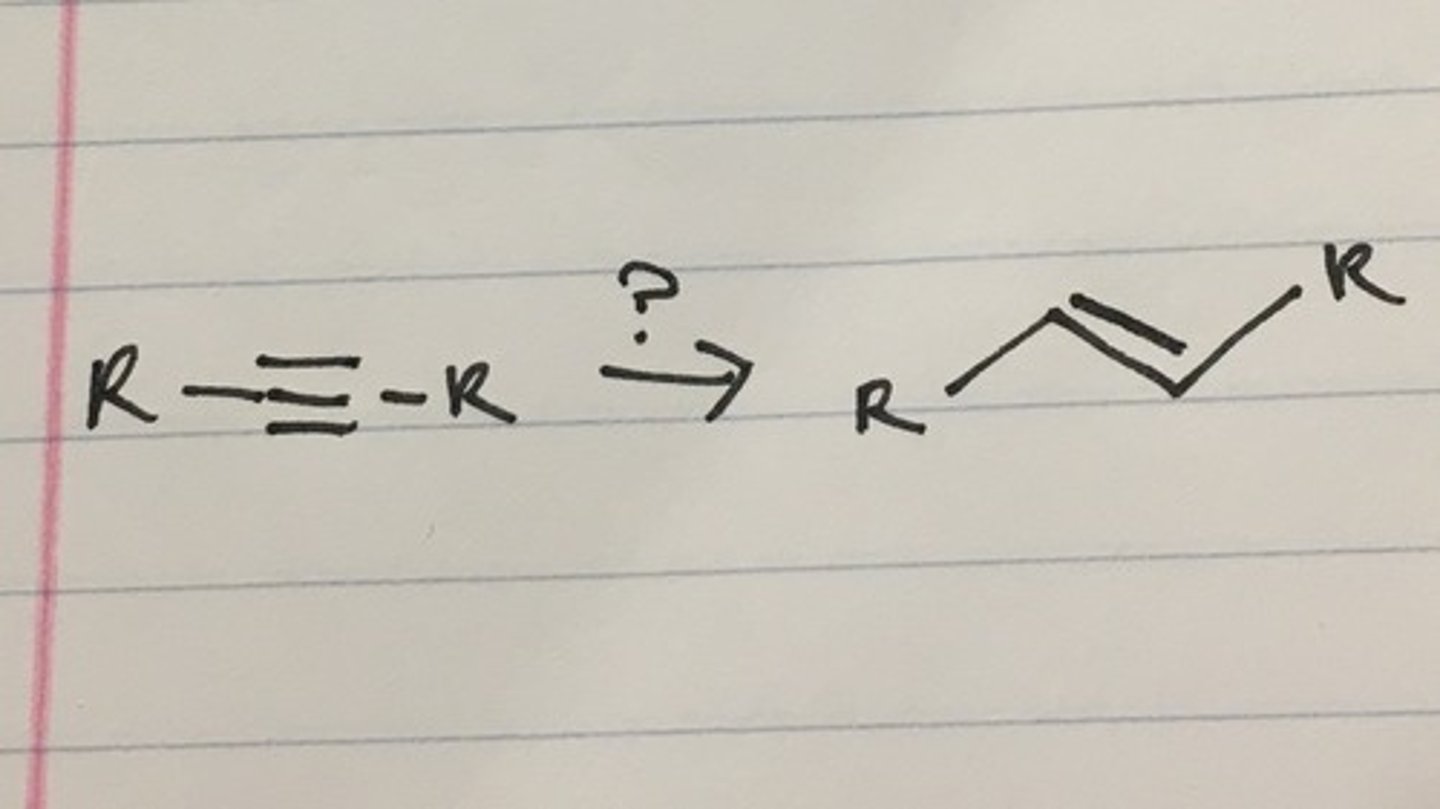
Alkyne goes through
HgSO4
H2SO4, H2O
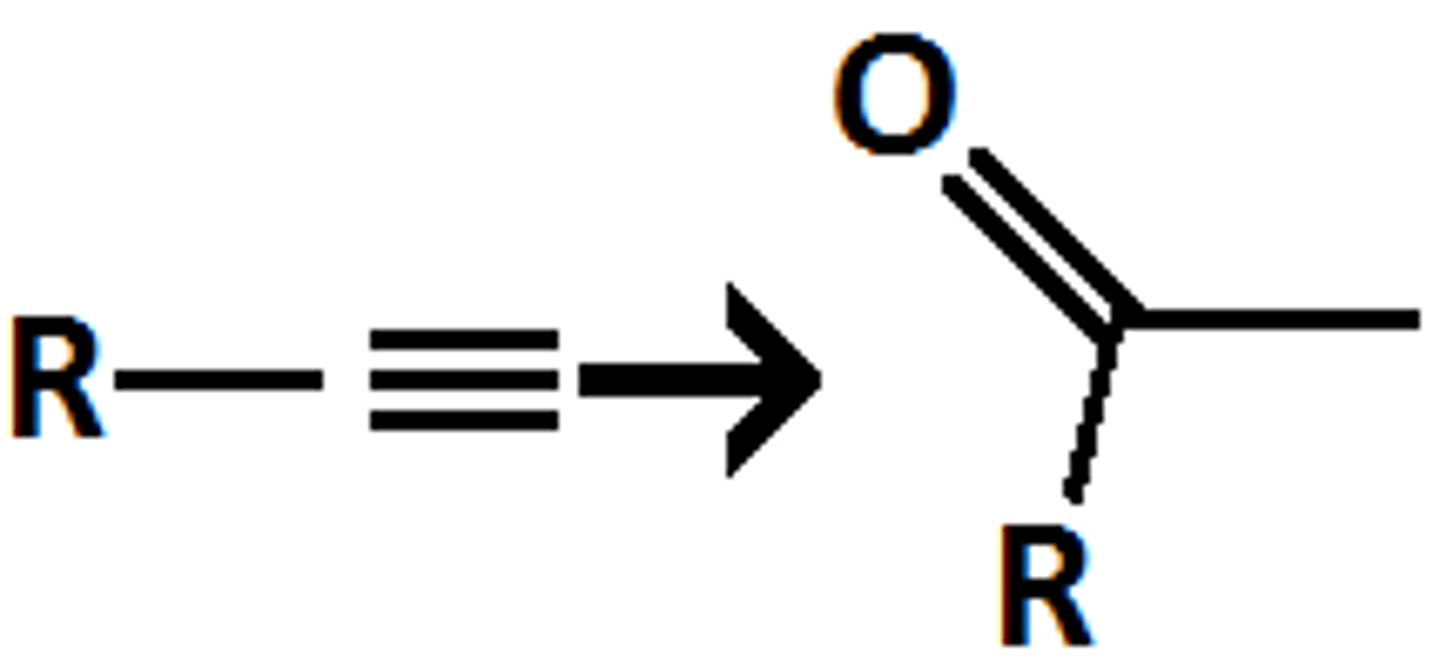
Alkyne goes through
9-BBN
NaOH, H2O2
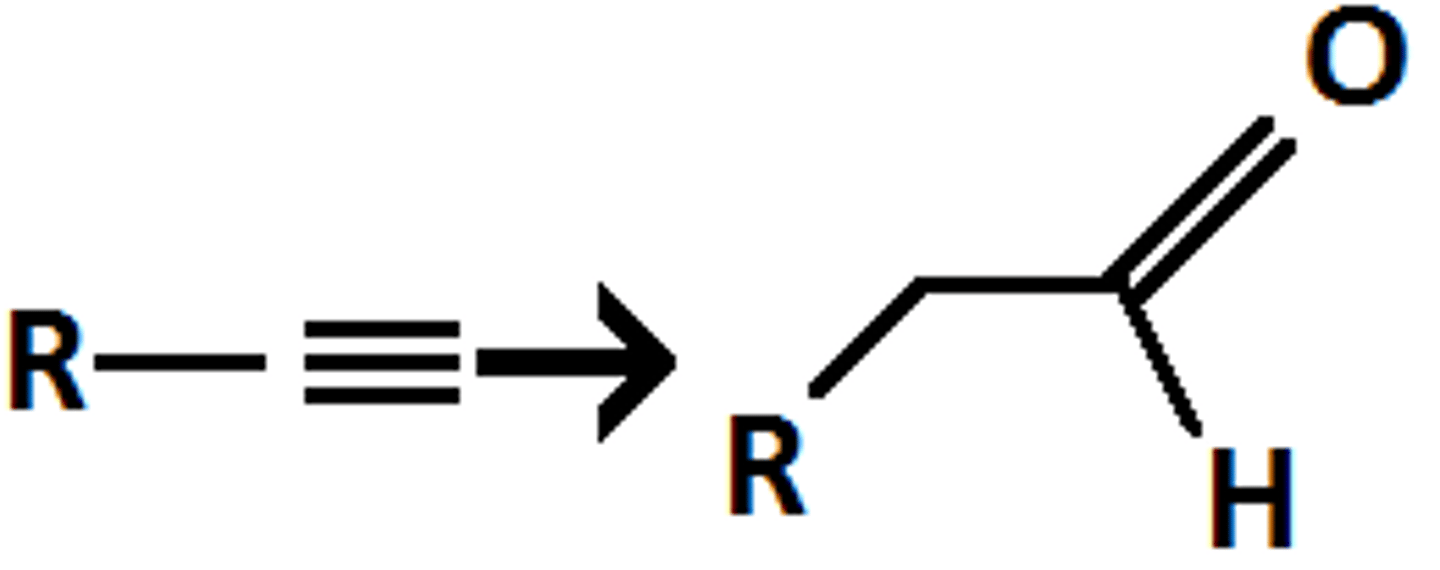
Alkyne goes through
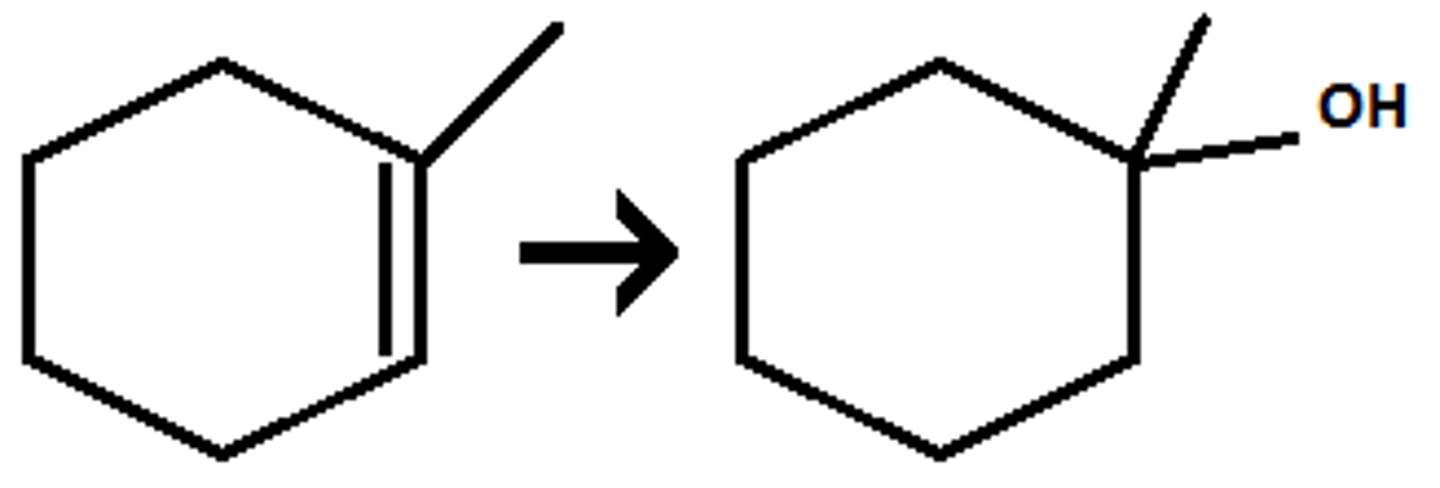
xs HX
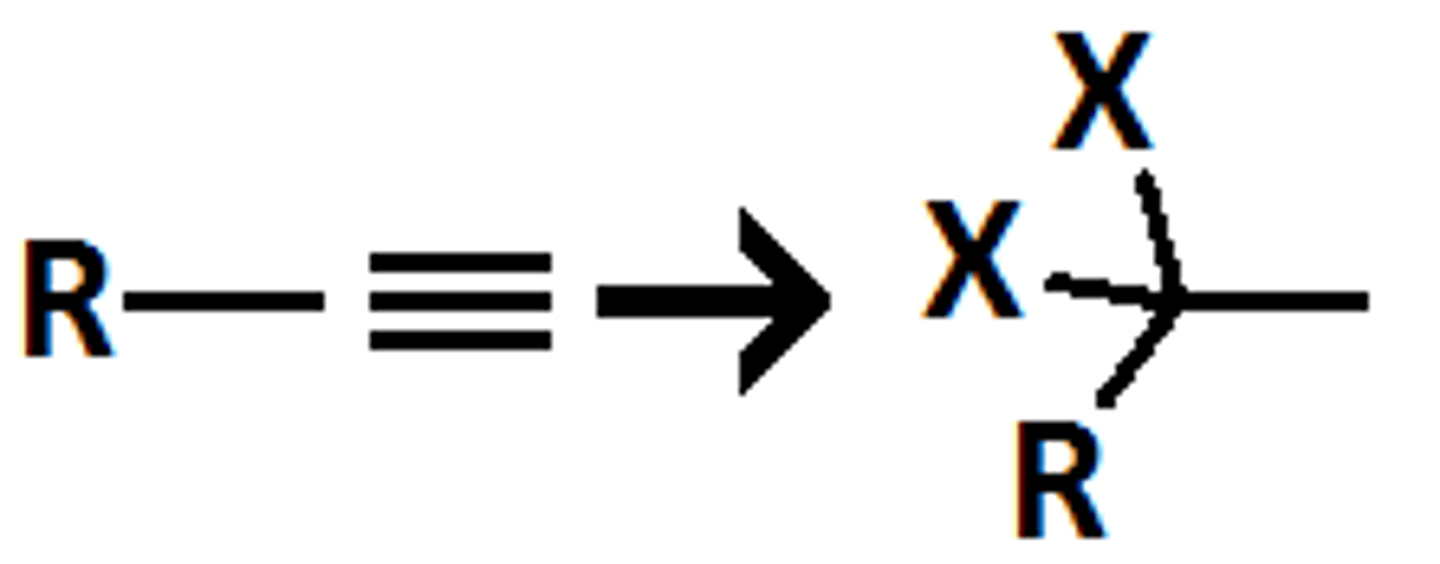
Alkyne goes through
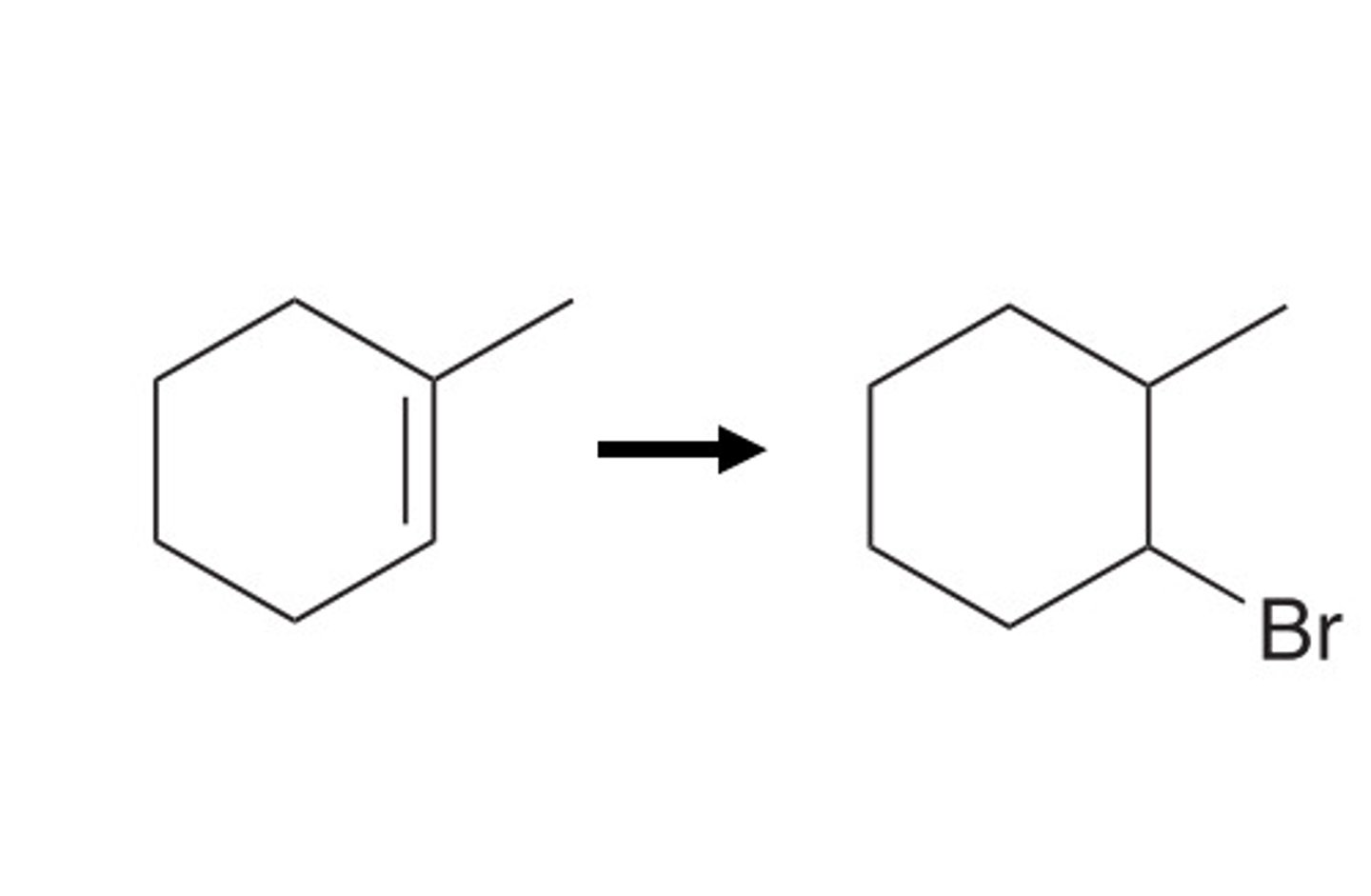
HX
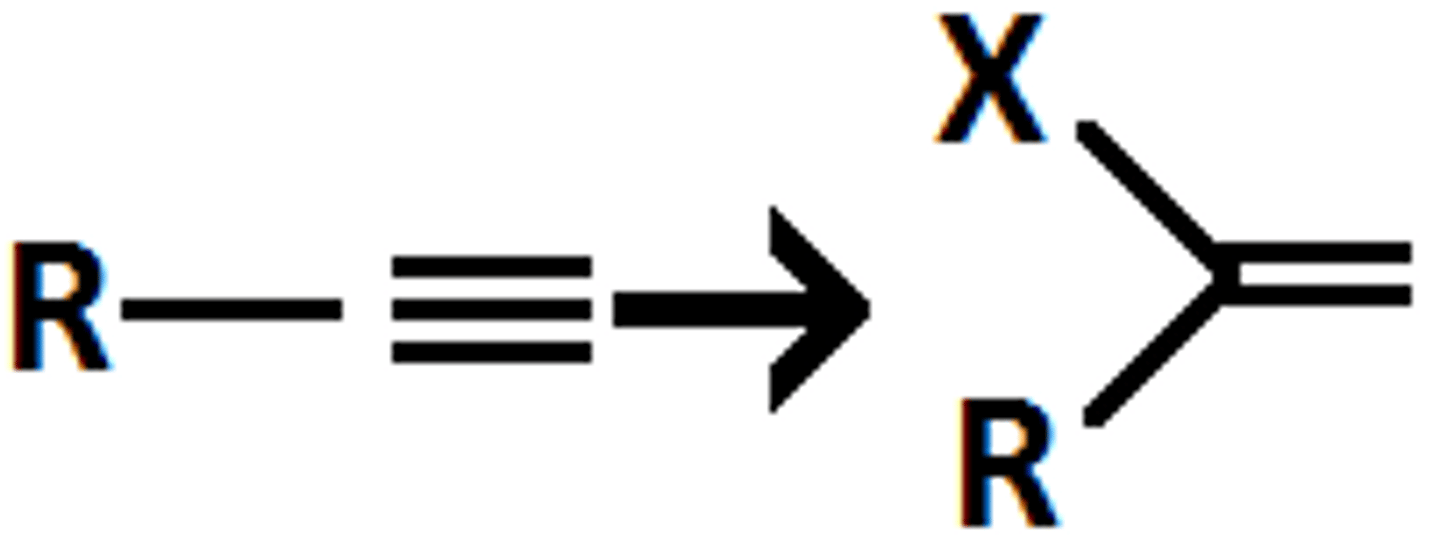
Alkyne goes through
X2
CCl4
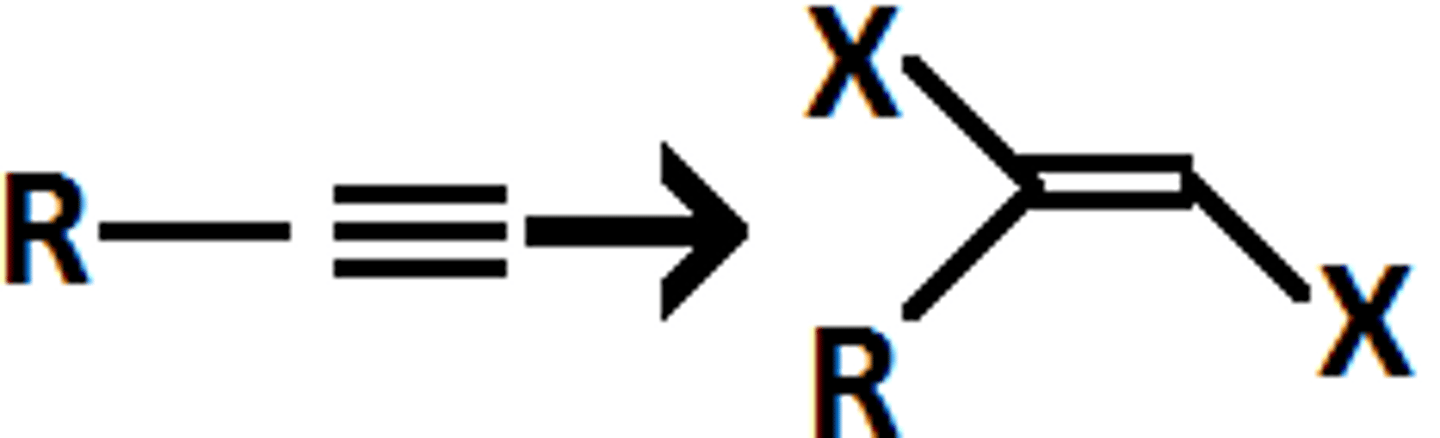
Alkyne goes through
xs. X2
CCl4
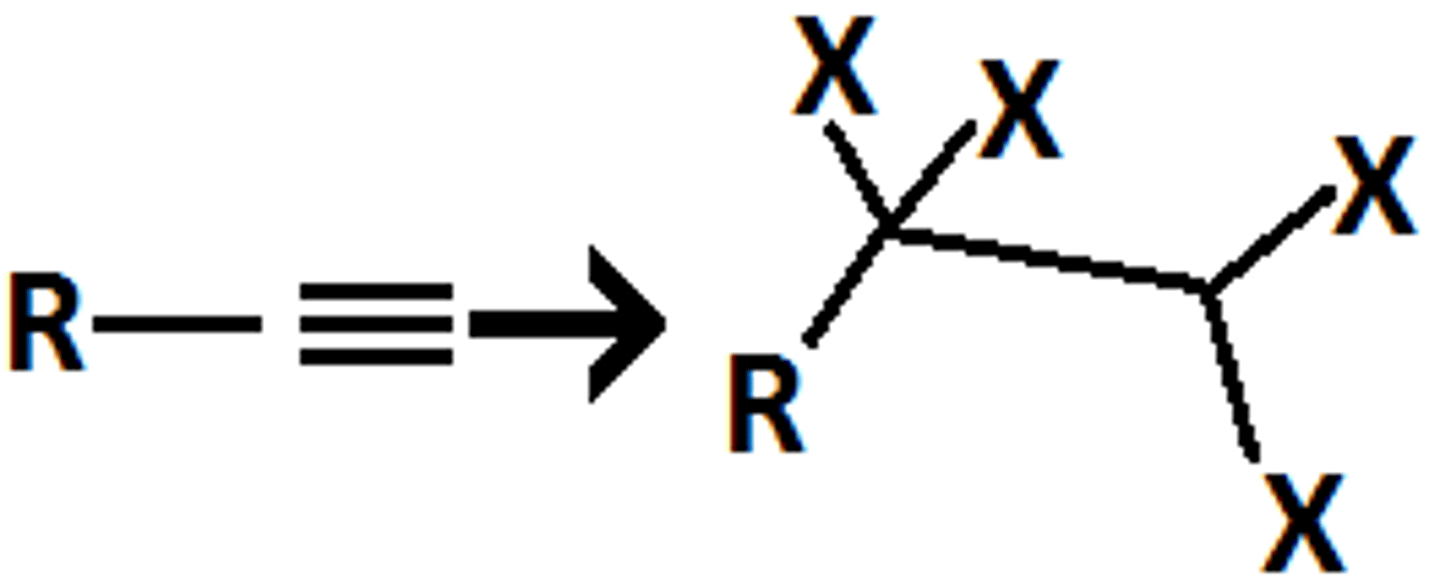
Alkyne goes through
O3
H2O

Alkyne goes through
NaNH2
RX
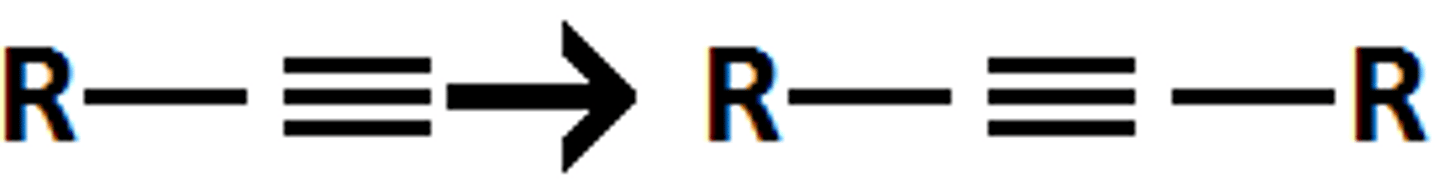
Ketone goes through
H2
M Catalyst
(if multiple double bonds, both get taken out, making it worse/ less selective)
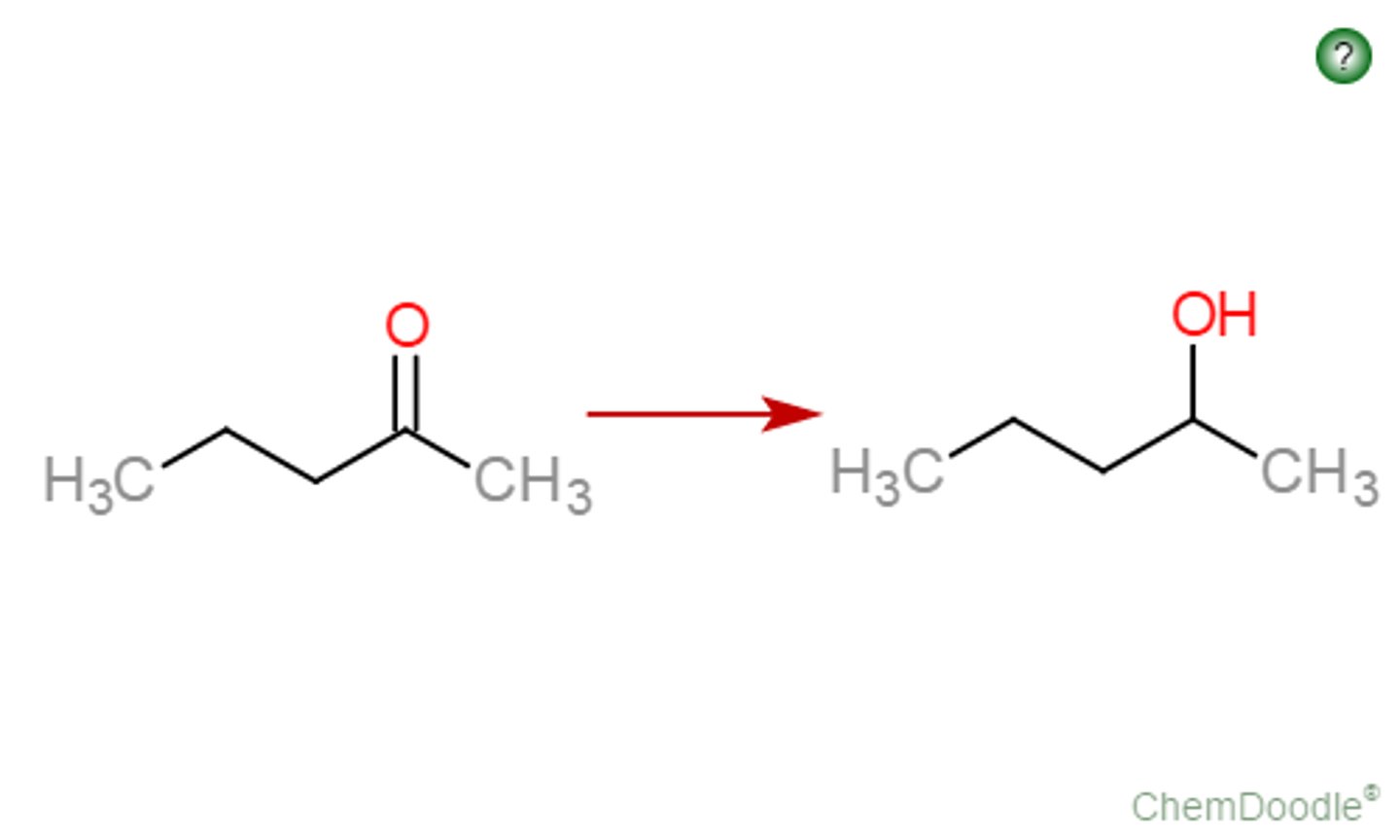
Ketone goes through
NaBH4 or LAH
(LAH better because it can do ester)
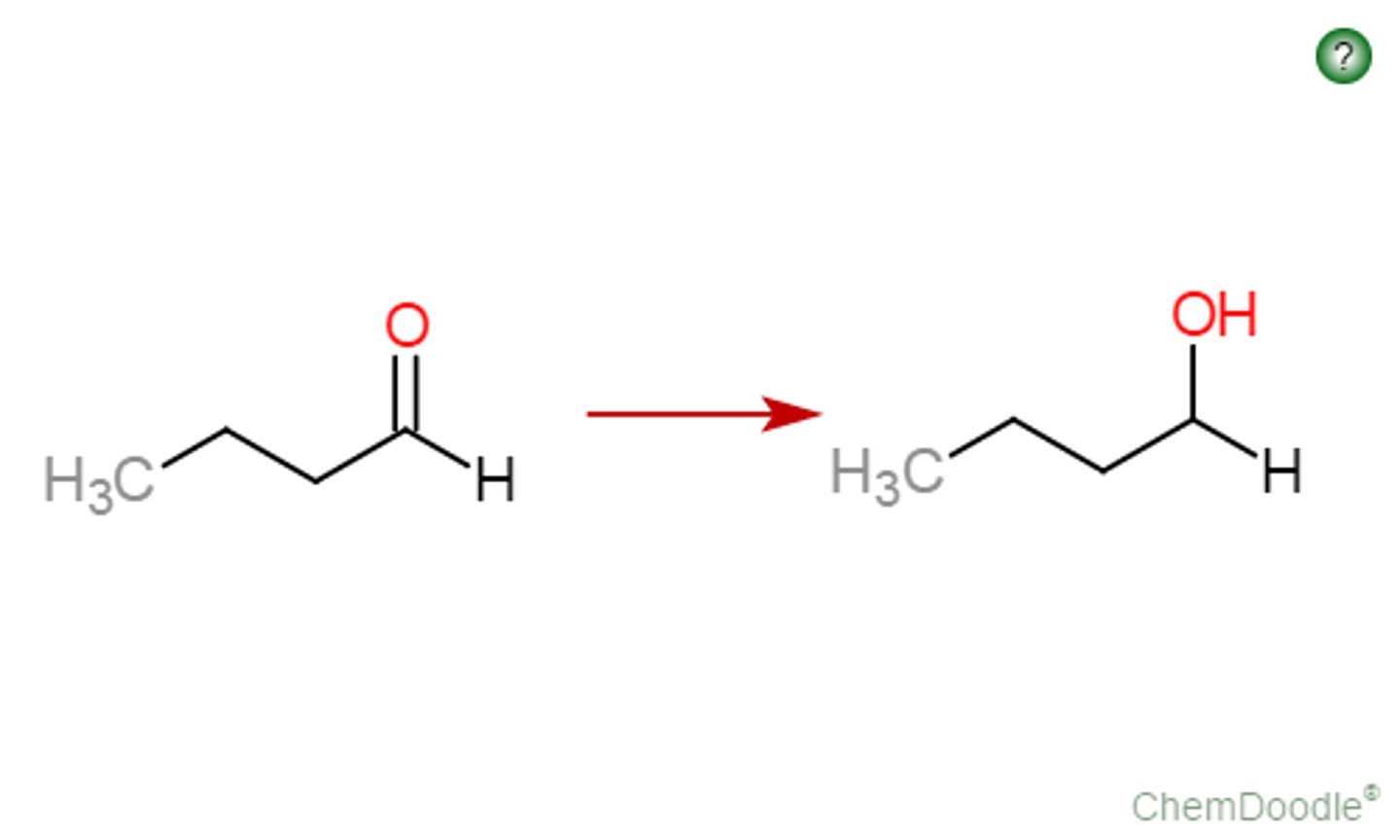
Ketone goes through
RMgX

Ketone with OH attached goes through
TMSCl, Et3N
Mg
Specefic Carbonyl
H2O
TBAF
Protection, adds specific carbonyl
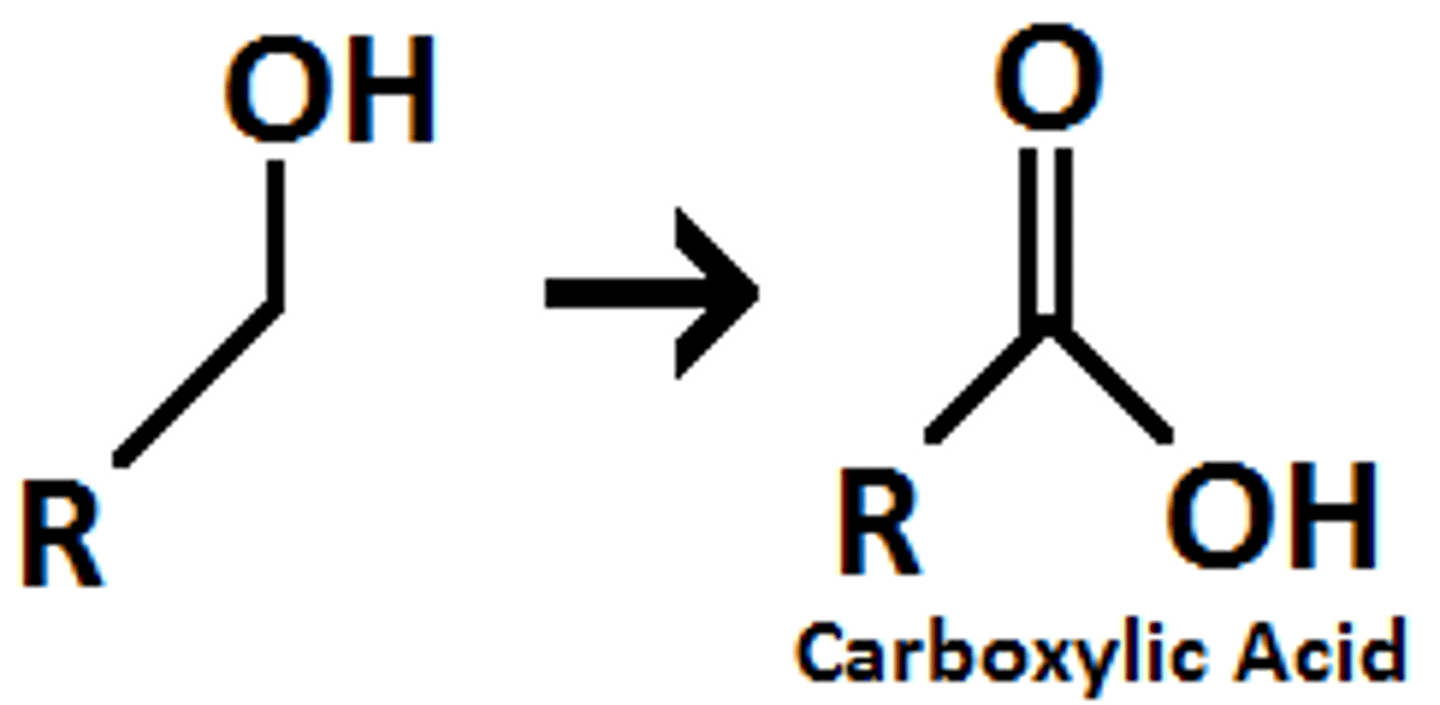
Alcohol goes through
Na2Cr2O7
H2SO4, H2O
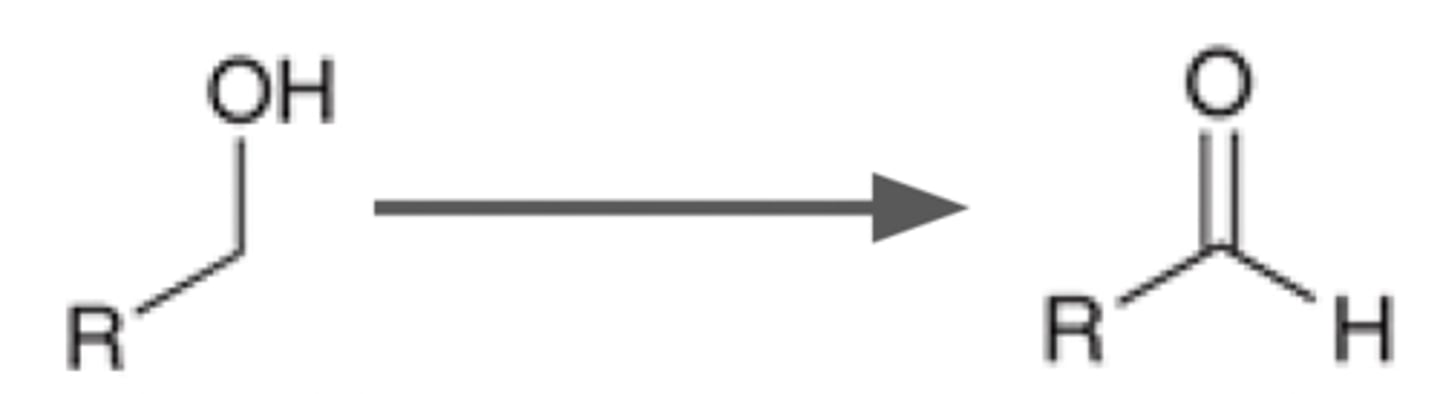
Alcohol goes through
PCC
CHCl2
or
DMSO, (COCl)2
Et3N
or
DMP
CH2Cl3
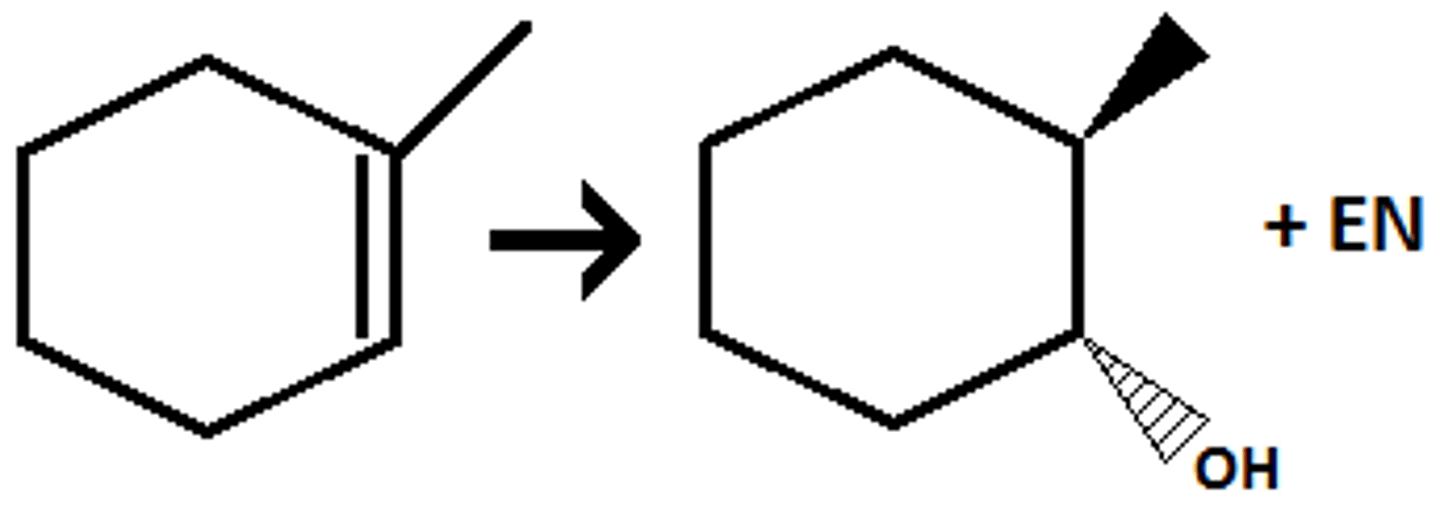
epoxide ring goes through
strong nucleophile
H20
Anti addition of OH/Nu, nuc attacks less substituted and O goes to more substituted
Epoxide Ring goes through
weak nucleophile (H2SO4/MeOH,HBr)
anti addition of OH/nuc, nuc attacks more substituted side and O goes to less substituted
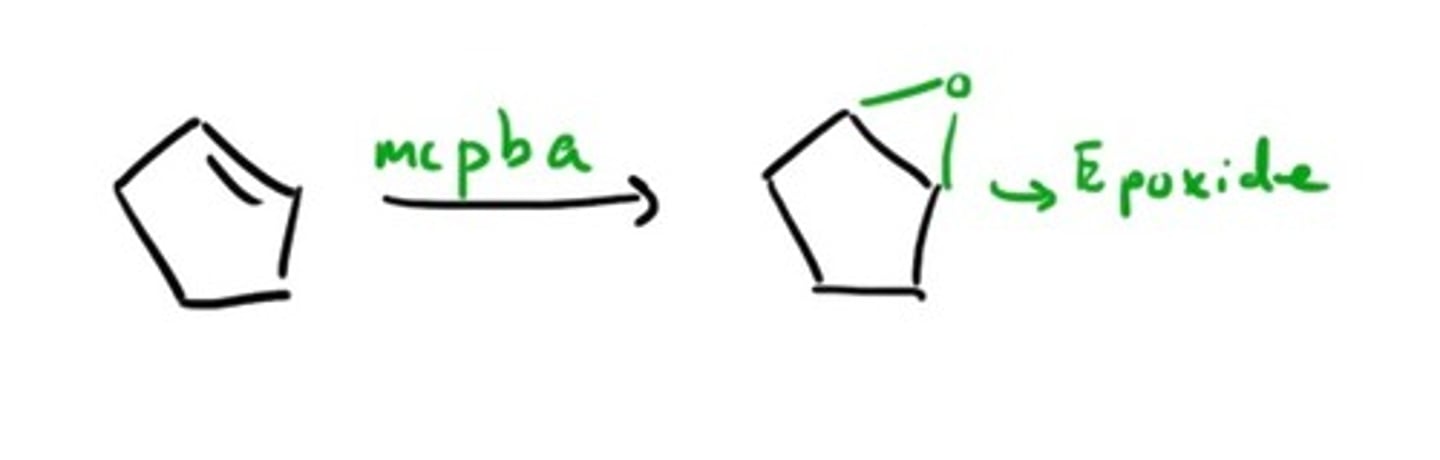
Double bond goes through
mCPBA
Alcohol goes through
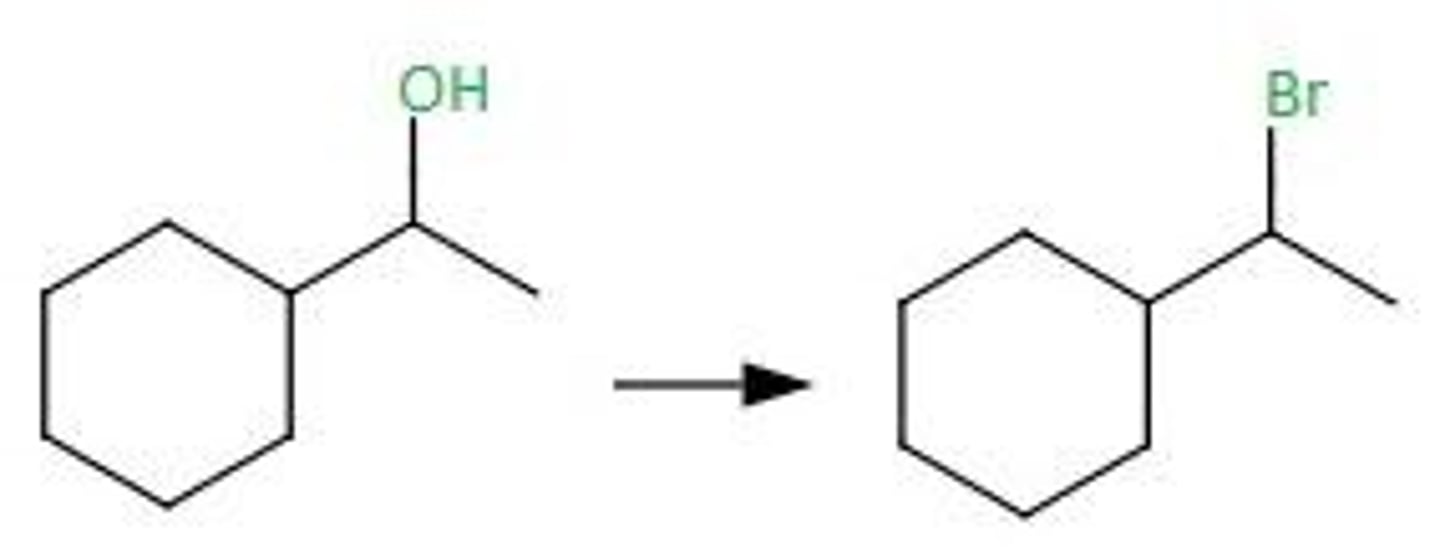
HX
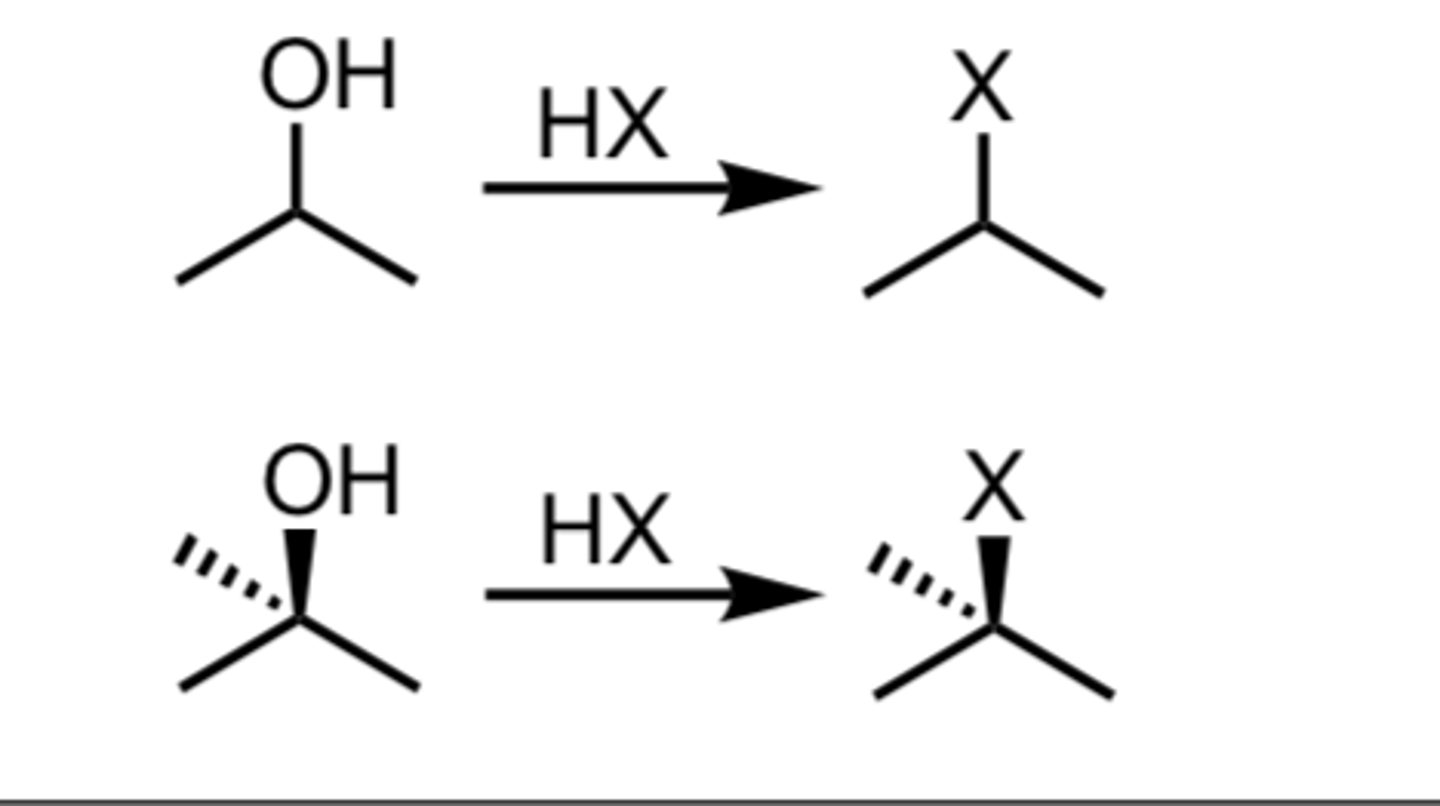
Alcohol goes through
HCl
ZnCl2
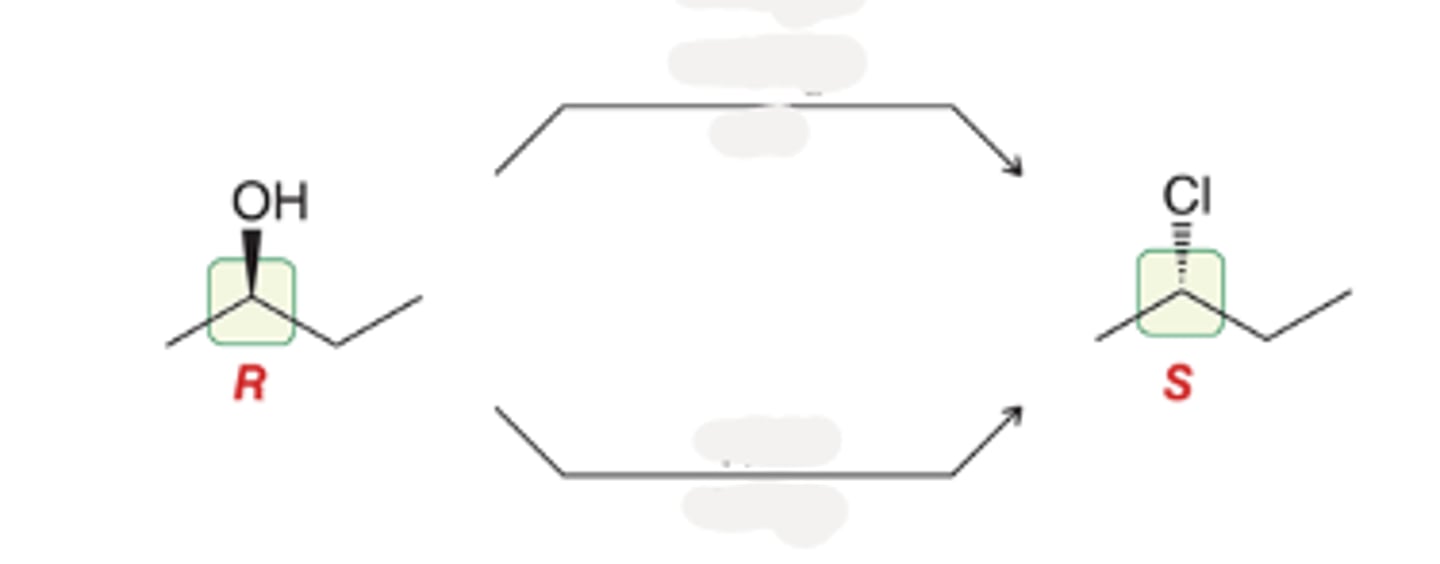
Alcohol goes through
SOCl2
Py
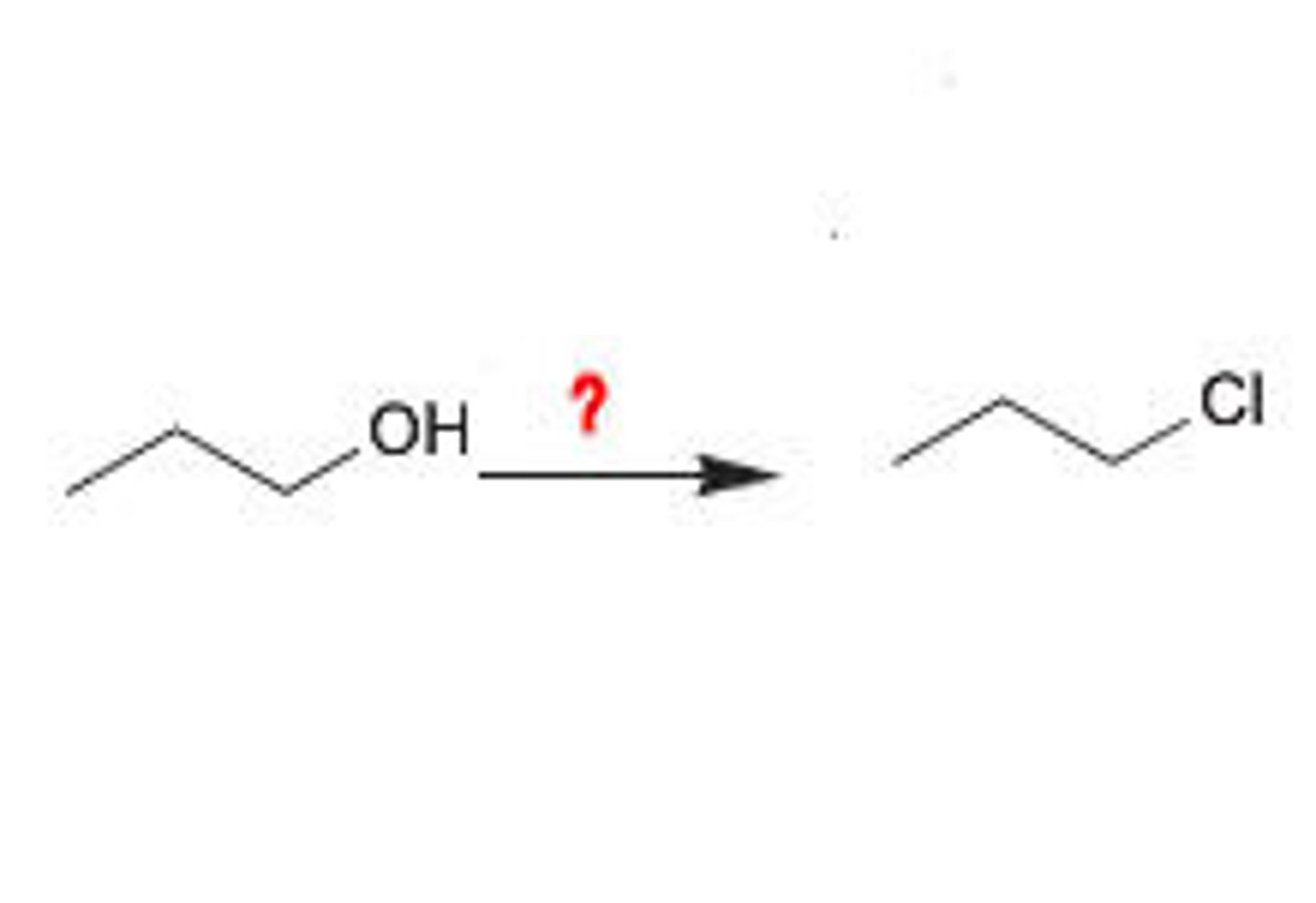
Alcohol goes through
PBr3