VSEPR Chemistry (11)
0.0(0)
Card Sorting
1/10
There's no tags or description
Looks like no tags are added yet.
Last updated 5:50 PM on 1/4/26
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
11 Terms
1
New cards
NON POLAR COVALENT BONDING
electrons are equally shared between 2 atoms (no charges on atoms) EN: 0 - 0.4
2
New cards
POLAR COVALENT BONDING
electrons shared unequally between 2 atoms (partial charges on atoms) EN: 0.5 - 1.7
3
New cards
IONIC BONDING
complete transfer of 1 or more valence electrons (full charges on resulting ions) EN: 1.7<
4
New cards
IONIC BOND
electrostatic force of attraction between a positive ion and a negative ion
5
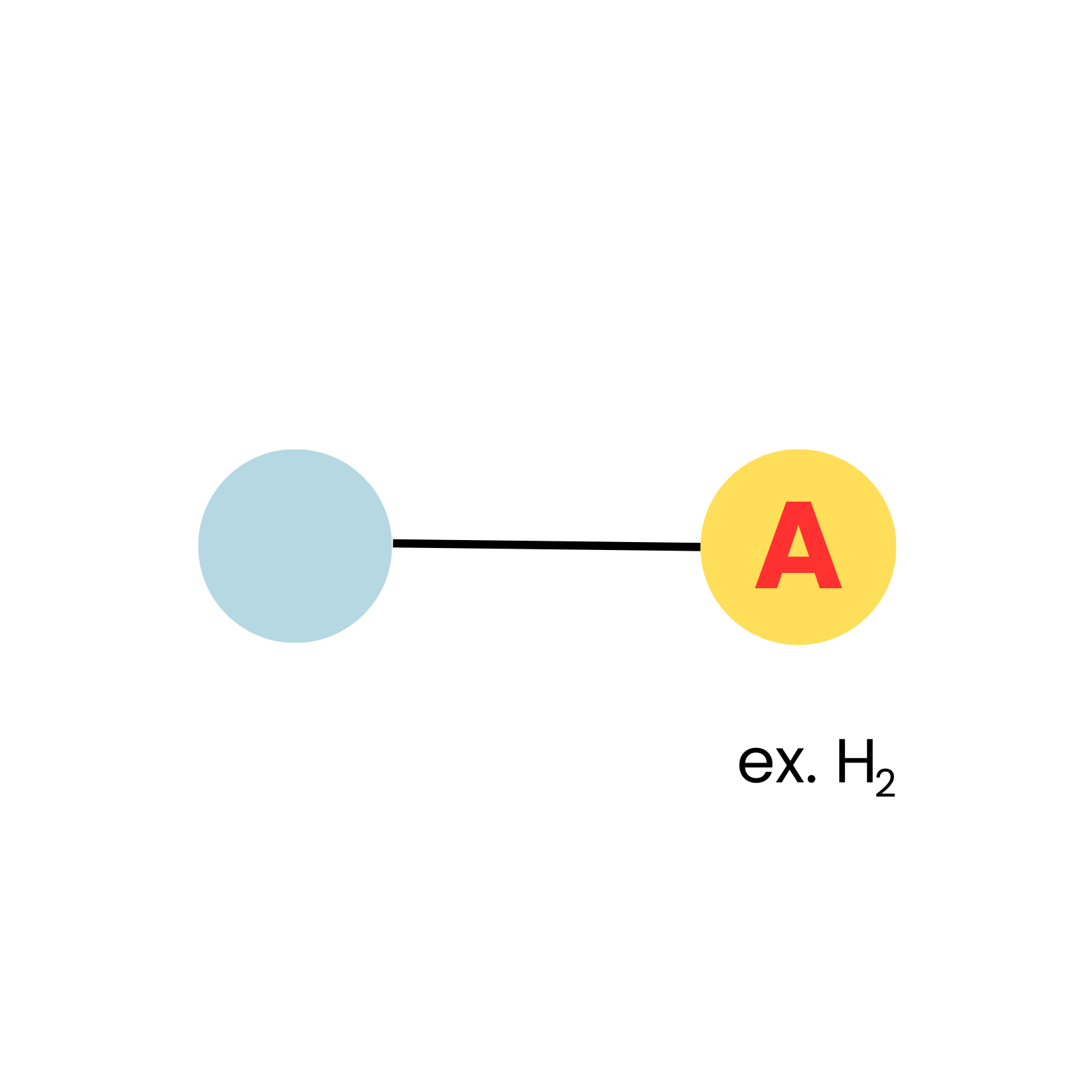
New cards
AX1
linear
6
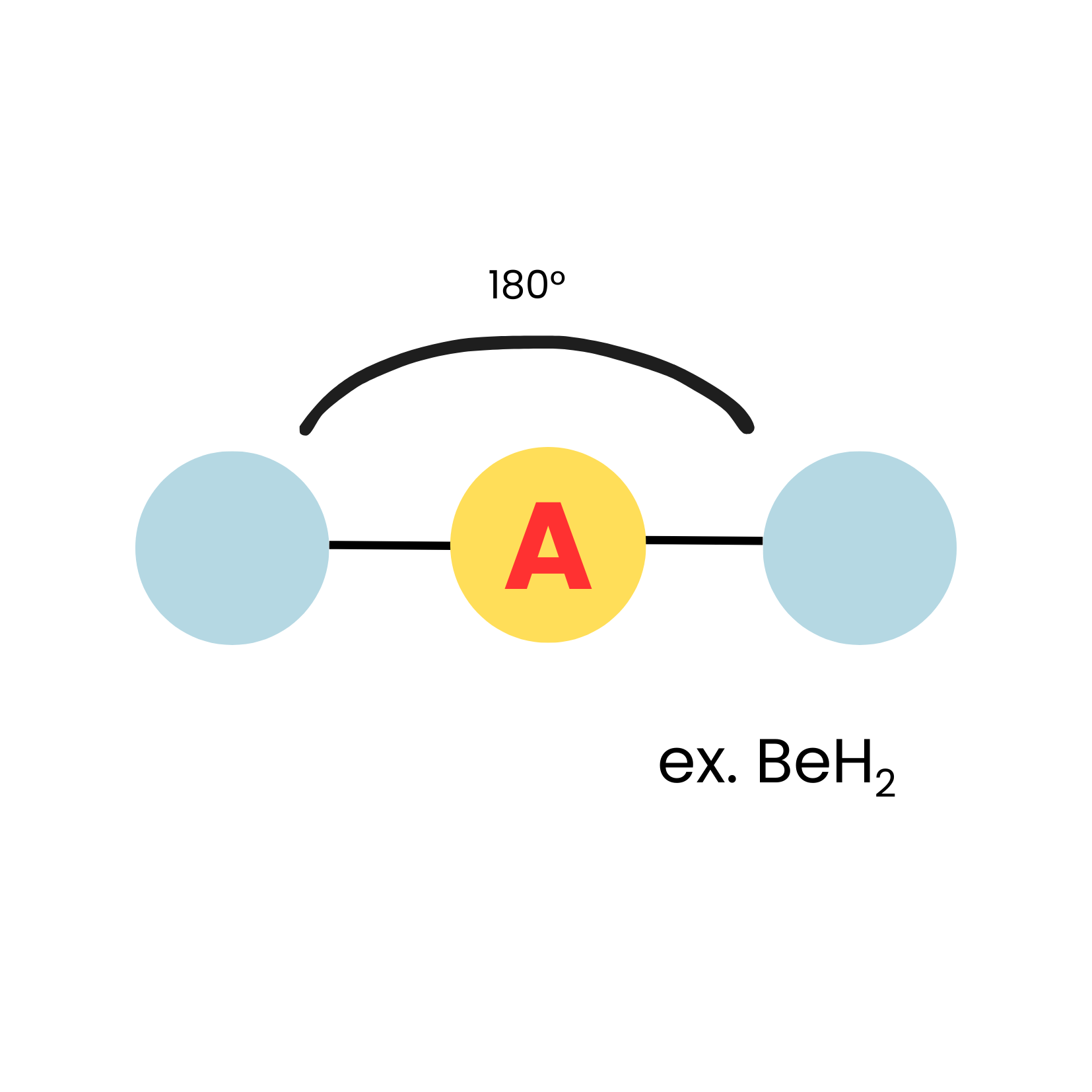
New cards
AX2
linear
7
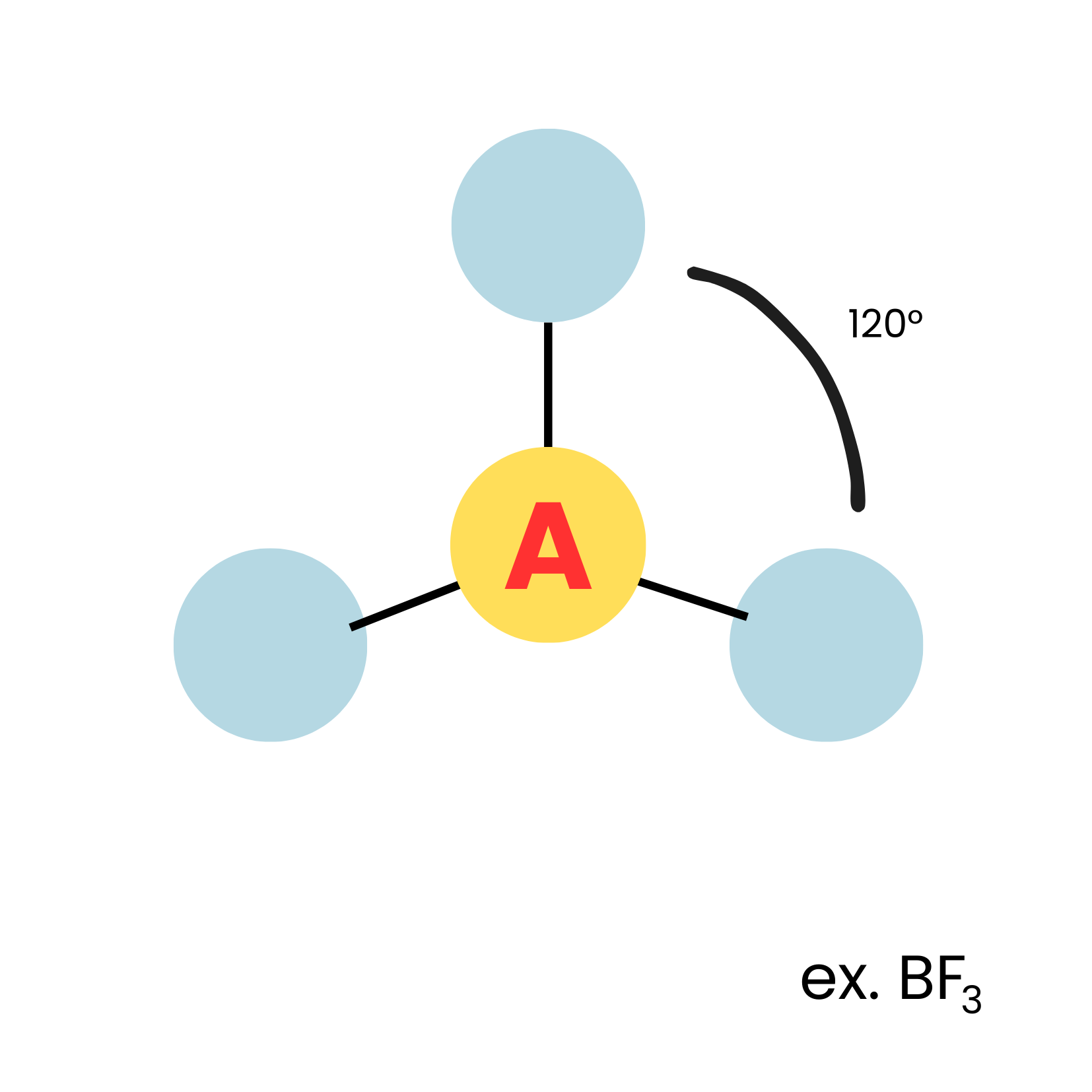
New cards
AX3
trigonnal plannar
8
New cards
AX4
tetrahedral
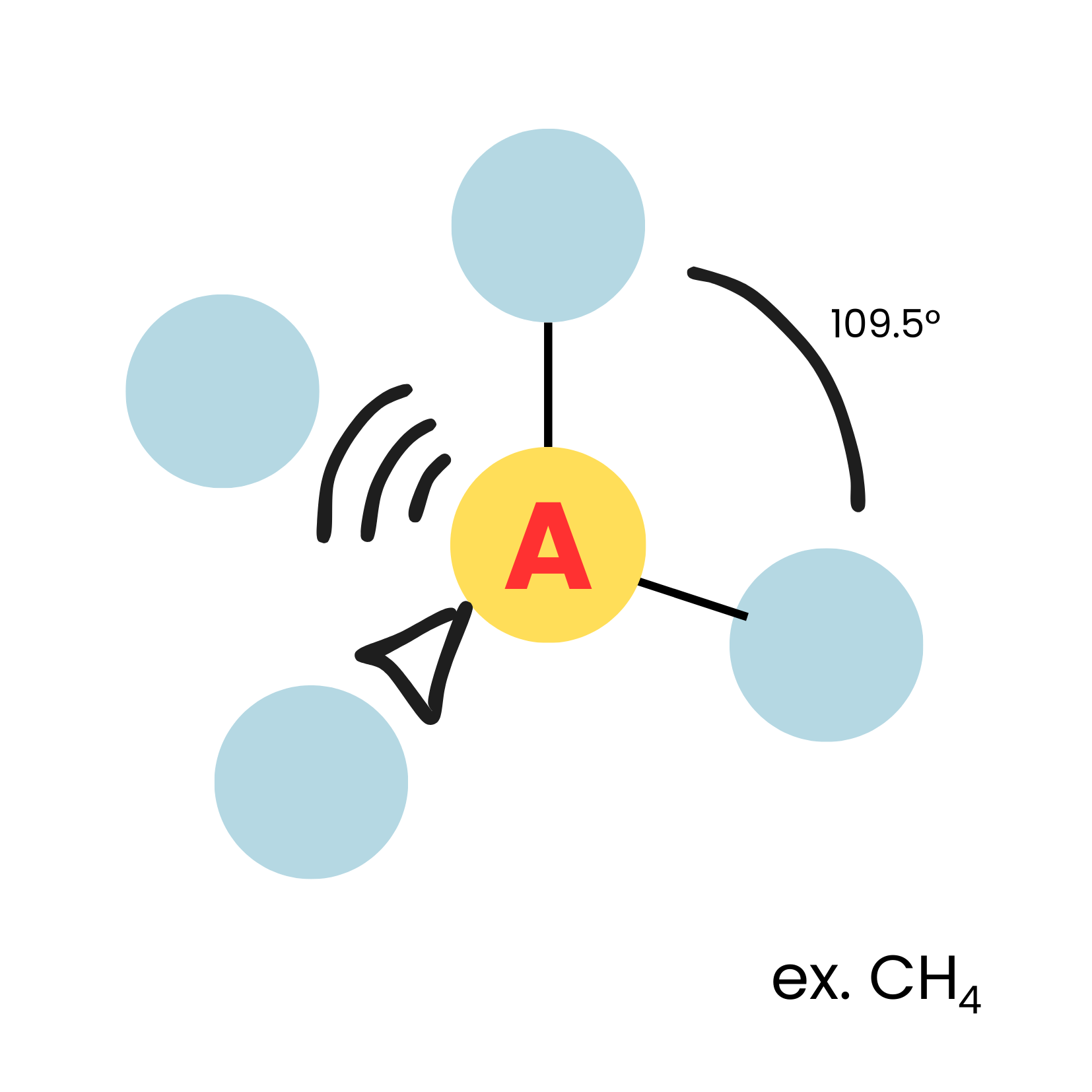
9
New cards
AX1E3
linear
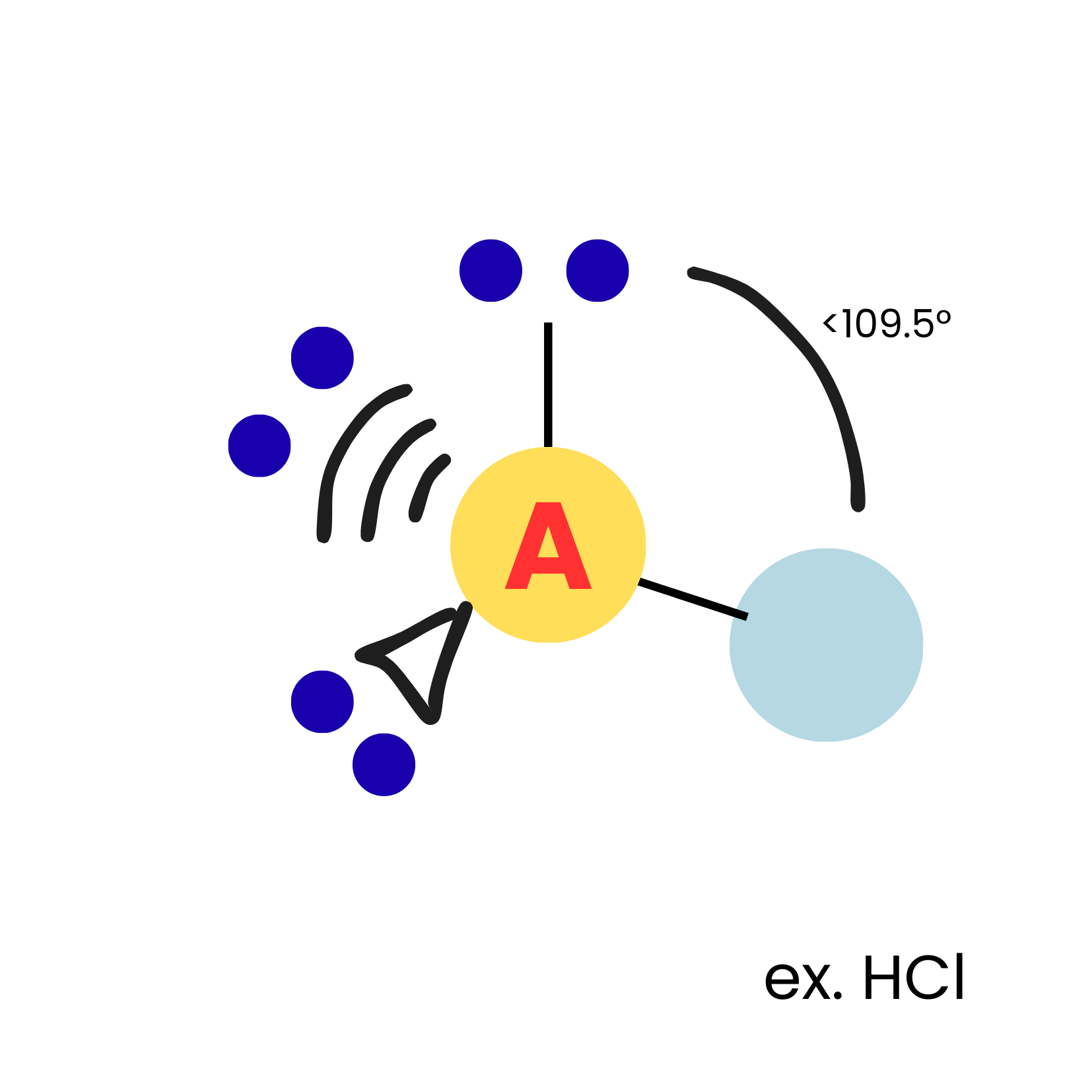
10
New cards
AX2E2
bent
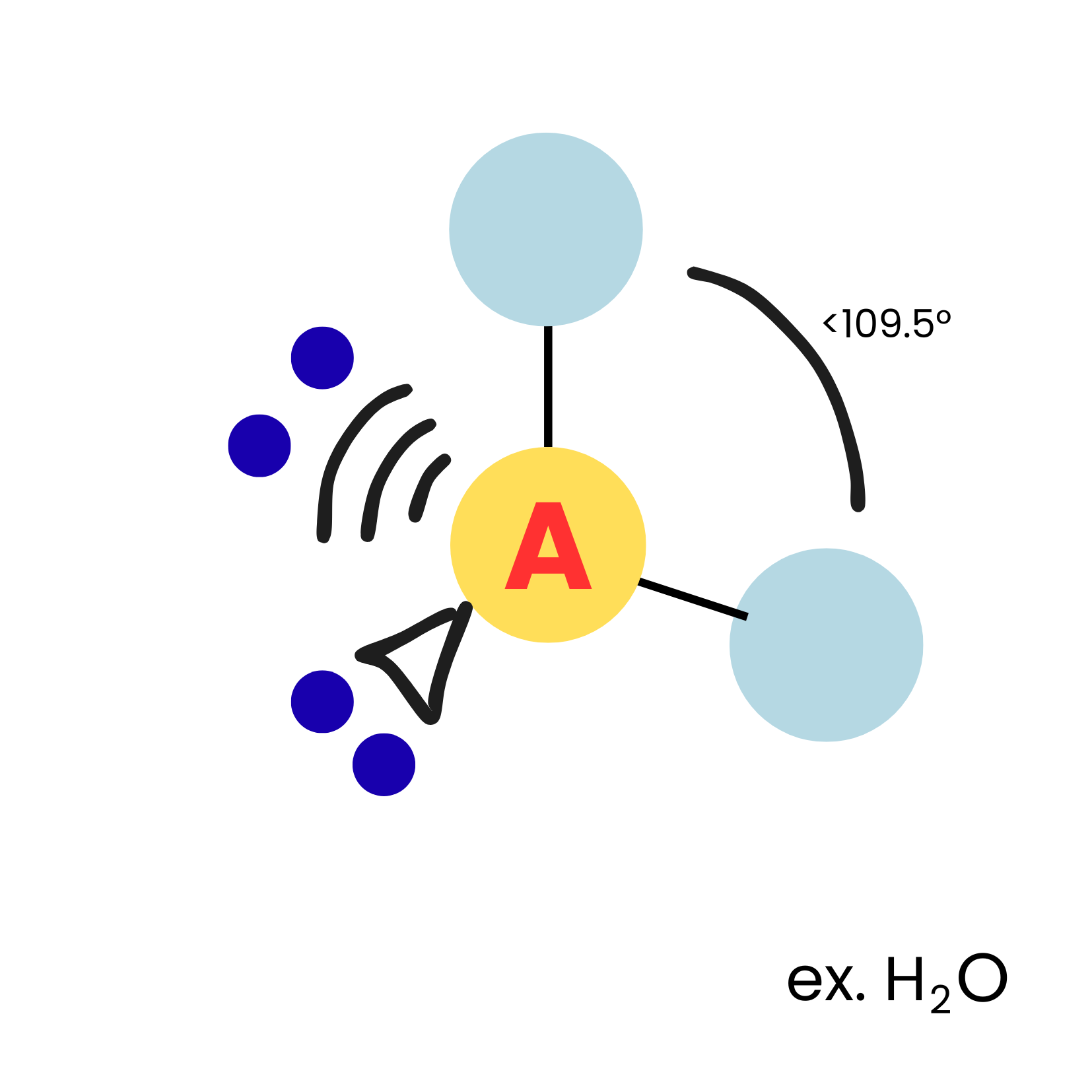
11
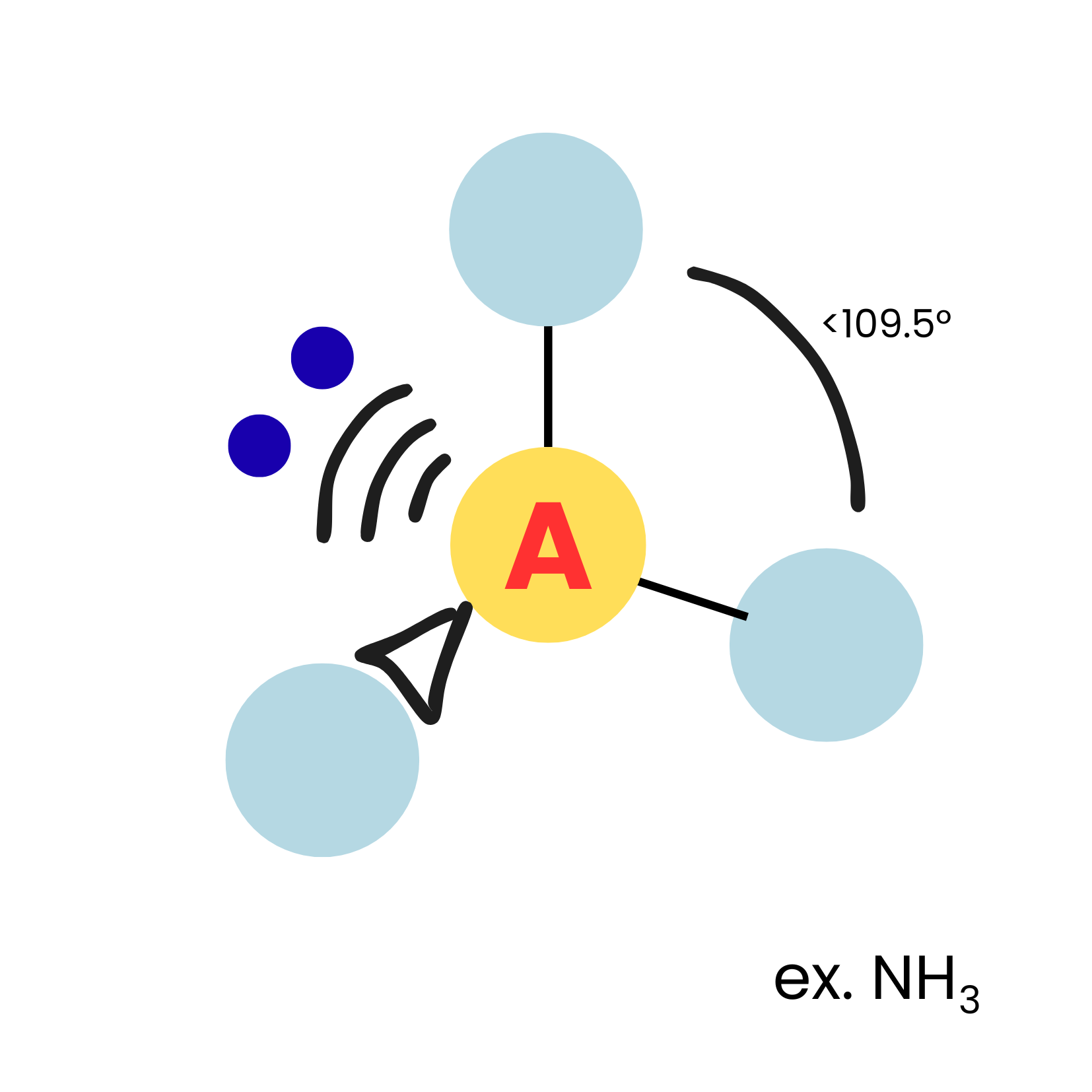
New cards
AX3E1
trigonnal pyramidal