8.4 - Depth Study
1/32
Earn XP
Description and Tags
COMPLETED - ALL OF 8.4
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
33 Terms
nucleon
collective name for protons and neutrons
atomic number
number of protons in an atom
mass number
number of nucleons in an atoms (protons+neutrons)
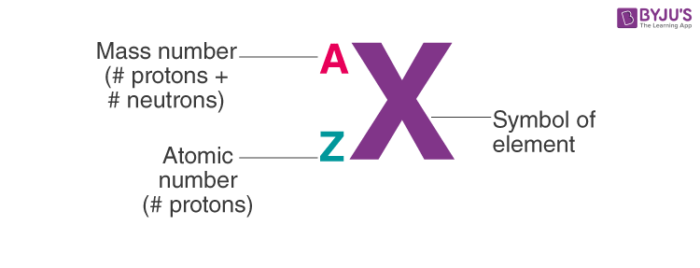
isotopic notation
Strong Nuclear Force
a force that keeps the nucleus together which only acts across small distances, occurs between nucleons
“nuclear glue”
range: 2.5 to 3 femtometers, where 1 fm is 10-15 meters
note: the force closer than 1 × 10-15 m is repulsive
nuclear stability
the tendency of a nucleus of an atom to decay
transmute
the process in which an atom decays and changes into something else (a different element)
radioactive
an unstable nuclide that undergoes radioactive decay
nuclide
Nuclides are a class of atoms characterized by their number of protons, Z, their number of neutrons, N, and their nuclear energy state — NOT SAME AS ISOTOPE, can be referring to same or different elements
predictions of nuclear stability
neutron to proton ratio
the band of stability
magic numbers
‘band of stability’
Found on graph of stability for elements of the periodic table. A nuclide falling in the band of stability is stable (not radioactive)
transmutation
the change of one chemical element into another by nuclear decay or radioactive bombardment
spontaneous radioactivity
radioactivity not caused by human intervention or accelerators
Ionisation
the removal of a bound electron from an atom to produce a free electron and a positive ion (THIS IS NOT RADIOACTIVE DECAY)
Radioisotopes
unstable atoms that undergo nuclear reactions (decay) to become more stable by emitting particles
version of a chemical element that has an unstable nucleus and emits radiation to become a more stable form
Decay series
Occurs when one radioactive isotope decays into another, but the daughter is also unstable and further decays occur until a stable nucleus is created
Radioactive Decay Law
Nt = N0 e-λt
where N0 = initial number of radioactive particles (t=0)
Nt = number of radioactive particles remaining after time (t)
λ = decay constant (per time unit → determined by sample etc)
Nuclear Fission
a heavy nucleus splits to form two or more lighter nuclei, each of which is more stable than the original nucleus
Moderator
a substance that slows neutrons down to the required speed to cause a nuclear fission reaction
e.g. heavy water, deuterium oxide, graphite rods (most economical)
critical mass
the smallest amount of fissile material needed for a sustained nuclear chain reaction
nuclear reactor fuel
e.g. enriched uranium-235 (BE SPECIFIC) or plutonium-239
enriched uranium-235 has 5-20% of the uranium-235 isotopes and the rest is uranium-239
Control rods
control rate of nuclear reaction by absorbing neutrons (this rate can be adjusted)
e.g. made of steel with boron or cadmium
coolant
controls reaction temperature, can extract heat energy from reactor
can be: water, heavy water, air, helium or liquid sodium
Shielding
protects humans from gamma rays produced during nuclear reaction. shielded by:
graphite and lead to reflect neutrons
thick wall of concrete to absorb gamma radiation
equivalence
used to describe mass and energy and how they can be interchangable
Law of conservation of mass-energy (mass-energy equivalence)
E = mc2 + kinetic energy
invariant mass
‘rest mass’ — total energy of an object is calculated by its rest mass and ‘increase of mass’ caused by an increase of kinetic energy when moving
relativistic mass
calculated by E = mc2 + KE
special theory of relativity
certain types of matter can be created or destroyed, but the total mass and energy associated with such matter remains unchanged in quantity
mass defect
binding energy is proportional to this mass difference
binding energy
the energy required to separate an atomic nucleus completely into its constituent protons and neutrons
when a nucleus breaks, binding energy is applied (to overcome the strong nuclear force holding it together)
when components form a nucleus, binding energy is released (lower energy)
fusion
two light atomic nuclei combine to form a single, heavier nucleus (TO BECOME MORE STABLE), releasing immense energy because the resulting mass is less than the original total, converting mass to energy
nucleosynthesis
the creation of new atomic nuclei from existing protons, neutrons, and lighter nuclei, primarily through nuclear fusion — occurs in stars