Orgchem - 5 Types of Hybrid Orbitals
0.0(0)
0.0(0)
Card Sorting
1/4
There's no tags or description
Looks like no tags are added yet.
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
5 Terms
1
New cards
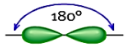
Shape: Linear
Hybridization of the central atom: sp
Number of Hybrid Orbitals: 2
2
New cards
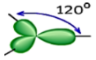
Shape: Trigonal Planar
Hybridization of the central atom: sp2
Number of Hybrid Orbitals: 3
3
New cards
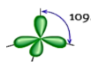
Shape: Tetrahedral
Hybridization of the central atom: sp3
Number of Hybrid Orbitals: 4
4
New cards
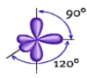
Shape: Trigonal Bipyramidal
Hybridization of the central atom: sp3d
Number of Hybrid Orbitals: 5
5
New cards
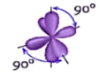
Shape: Octahedral
Hybridization of the central atom: sp3d2
Number of Hybrid Orbitals: 6