IMFs and State Changes- Chapter 10
1/36
Earn XP
Description and Tags
Nov 17 and 19 Lectures
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
37 Terms
Ideal Gas Law Assumptions
There are no (or entirely negligible) intermolecular forces between the gas molecules
The volume occupied by the molecules themselves is negligible
Intramolecular Forces
covalent and ionic bonds
provide structure and shape
Intermolecular Forces
Forces between molecules
Responsible for condensed phases (aka liquids and solids)
are covalent or ionic forces stronger when intramolecular?
ionic
are inter or intramolecular forces stronger?
intramolecular are stronger
types of intermolecular forces
ion-dipole
dipole-dipole
hydrogen bonding
London dispersion forces
ion-dipole forces
ionic solid dissolves in water (or any polar substance)
dipole-dipole forces
between two polar molecules (such as water)
regions of partial positive charge are attracted to regions of partial negative charge on neighboring polar molecules
Hydrogen bonding
Extreme dipole-dipole
H-N
H-O
H-F
Why is water special, with a high BP?
The O in H2O has two lone pairs, so can H-bond with two other water molecules
volatility
the tendency of a substance to vaporize or evaporate
vapor pressure
in a closed system, the equilibrium pressure where there is a dynamic equilibrium between liquid and vapor
polarizability
how easily a molecule’s electron cloud can be distorted
larger e clouds= more polarizable= stronger IMFs
larger e clouds are squishy, easier to distort
Dipole-Induced
Polar molecule inducing a temporary dipole charge in a nonpolar molecule
London Dispersion Forces (weakest force)
One atom’s positive nucleus is attracted to the other’s electrons, and vice versa
Competing forces cause uneven electron distribution, causing temporary induced dipoles
Occur between all molecules!!
the weaker the attractive forces, the ___ the vapor pressure
higher
aka more volatile
vapor pressure increases with an increase in ____
temperature
clausius clapeyron equation
Pvap= mmHg
C= constant characteristic of the liquid you’re using
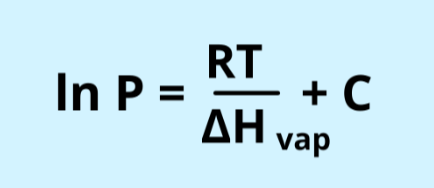
vaporization (evaporation)
gas in equilibrium with liquid (of same compound)
bolzmann distribution main idea
at higher temperatures, more molecules tend to be higher in energy to the point where their energy overcomes that of the intermolecular forces (vaporization)
Average energy depends on temperature
-∆H of condensation is the same as
∆H of vaporization
measured in kJ/mol
condensation is ____ thermic
condensation is exothermic
being a gas requires more energy (heat) and you give that up when you condensate
vaporization is ____thermic
endo
this is why we sweat!! “evaporative cooling
think of it as swallowing the heat E around in order to “fuel” evaporation
boiling point definition
when vapor pressure = external pressure
therefore, boiling point increases with increased pressure
critical point
above the critical point, the interface between liquid and vapor disappears
supercritical fluid- density like a liquid, viscosity like a gas
“green solvent” (little footprint) used to extract caffeine from coffee beans
melting point definition
temperature at which solid is converted to liquid
∆H of fusion is equal to the opposite of ∆H of melting
∆H fusion
enthalpy change that occurs at the melting point when a solid melts
magnitude of ∆H fusion depends on what is holding it together
sublimation
solid to gas
general solubility rule
“like dissolves like”
you need a solubility table for determining which ___ compounds are soluble
ionic
SO IMPORTANT!!! a polar bond has a minimum electronegativity difference of
0.4
are larger or smaller molecules more soluble
smaller
what makes something most soluble in water
polar
can form H-bonds with water
smaller molecule
Henry’s law
gas solubility increases with increasing pressure
S∝P
gas solubility factors
solubility increases when pressure increases (Henry’s Law)
solubility decreases with increasing temperature (the kinetic energy of molecules increases. Therefore, more gas can escape from solution)
Capillary Action
the ability of a liquid to flow up a narrow tube unassisted against gravity, is the result of cohesive and adhesive forces. When the adhesive forces between the liquid and the narrow tube are greater than the cohesive forces between the liquid molecules, the liquid molecules in contact with the wall of the tube are drawn up the side of the tube. The cohesive forces in the liquid cause the liquid molecules not in contact with the tube walls to be pulled up the tube as well. The liquid rises in the tube until the capillary action is balanced by the force of gravity. In liquids in which the cohesive forces are greater than the adhesive forces, capillary action does not occur.
Surface Tension
Surface tension is the result of a liquid attempting to minimize its surface area. The molecules at the surface of a liquid have fewer neighboring molecules to interact with than molecules in the interior of the liquid which are surrounded by molecules on all sides. Because there are only molecules to the side and below the surface molecules, the intermolecular forces pull the molecules at the surface downward into the bulk of the liquid. This downward pull minimizes the surface area and results in an elastic‑like surface. The surface tension decreases as the intermolecular forces decrease.