NBE Studying: Thanatochemistry Review
1/584
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
585 Terms
what is the most common solvent used to dilute embalming fluid?
water
what is the main participant in the process of decomposition known as hydrolysis?
water
what is the molecular mass of water?
18 amu
what is hydrogen bonding?
an attraction to each water molecule to another water molecule
what does hydrogen bonding and the polarity of compounds create in our body?
all possible chemical reactions
what are the most prevalent hydrogen bonds?
Between H and O
Explain some of the physical properties of water
Able to withstand a significant addition of heat before the temperature raises, protects our bodies from temperature changes
What water-based phenomena is due to hydrogen bonding?
Surface tension
T or F: Frozen water is less dense than it’s liquid form
TRUE
what are hydrates?
salts with water combined into its crystal structure in a definite ratio
what are desiccants?
salts that hydrate readily with water that is in the atmosphere
what is a deliquescent?
salts that hydrate into the point of dissolving into a liquid
what does anhydrous mean?
hydrated salts that are heated to the point of having their water removed
what does efflorescent mean?
hydrated salts that give up their water to the atmosphere upon standing
what is the hydrate gypsum used for?
filling in the skull following a cranial autopsy
T or F: hydrolysis is not a chemical reaction with water
FALSE, it is
What creates hard water?
dissolved ions (chloride and sulfate salts)
What are some issues caused by hard water?
buildup of deposit on faucets, diminished soap cleaning effectiveness, coagulates and clots blood making embalming difficult
How do embalmers fix permanent hard water?
mask the effects through specific sorts of embalming fluids, remove the ions
what is solubility?
amount of solute that will dissolve in a specified amount of solute?
what is the liquid version of solubility?
miscible vs. immiscible
what is solubility determined by?
nature of solute and solvent
what are other factors that influence solubility?
mixing, surface area, temperature
what is a dilute?
well below solubility point
what is concentrated?
very near or at solubility point
what is unsaturated?
any concentration before solubility point
what is saturated?
at the solubility point
what is supersaturated?
beyond solubility point
what is the wt% formula by mass?
mass of solute (g)/mass of solvent (g) x 100
what is wt% by volume?
mass of volume (g)/volume of solution in (mLs) x 100
what is the formula for ppm?
(g) solute/(g) solution x 1,000,000
what is the definition of the formaldehyde index?
amount of formaldehyde gas (in mass or grams) present in 100 mLs of solution (ex. 20 index = 20g formaldehyde/100 mL of water)
T or F: the properties of solutions are different than their corresponding pure solvent
true
what is the macro effect?
solutes depressed through melting and boiling points
what is osmosis?
diffusion through a membrane
what does isotonic mean?
two solutions of equal concentration
what does hypertonic mean?
more concentrated than it’s partner
what does hypotonic mean?
less concentrated than it’s partnerwh
what is osmotic pressure?
pressure necessary to stop osmosis
if DEHYDRATED, bodies need…
hypotonic fluid
if EDEMATOUS, bodies need…
hypertonic fluid
what size are colloids?
under 100 nm, will pass through filters
what size are suspensions?
over 100 nm, will not pass through filter or membranes
what is the pH of blood?
7.35
humans are slightly….
base
What can be found in dissolved water?
Acids and bases
Describe the self-ionization of water
two water molecules will ionize creating two ions (hydroxide is basic at OH- and hydronium is acidic at H30+) - self-ionization is reversible and only happens in small amounts until equilibrium is reached on both sides
what are some properties of acids?
litmus paper turns red, reacts with base to form water and salt, reacts with carbonates and bicarbonates to create CO2, H20 and salt - can exist in all three states
how can acids create CO2 and H20 after reacting with carbonates/bicarbonates?
a chemical reaction where hydrogen ions displace carbon atoms in the carbonates, causing CO2 release AKA bubbles
what are some properties of bases?
litmus paper turns blue, slippery/soapy, neutralizes acids, can be found in all physical states but is typically a slippery solid
what is an example of a base neutralizing an acid?
dawn dish soap (base) used to neutralize cavity fluid (acid)
what is Arrhenius theory?
in water, acids are substances that produce hydrogen ions and bases (in water) produce hydroxide ions
what is Bronsted Lowry theory?
acid is a substance that donates a hydrogen proton to any substance, while bases are proton acceptors
what is G.N Lewis theory?
acid is defined as anything that accepts a pair of electrons, while a base is defined as anything that donates a pair of electrons
what does amphoteric mean?
a substance that can act as a base or an acid (ex. water acting as an acid in the presence of a base and acting as a base in the presence of an acid, also amino acids)
how is acid strength determined?
percentage that a substance dissociates in aqueous solution (strong is 60% and upwards, weak is 10% and downwards)
how is base strength determined?
percentage of a substance that dissociates into positive and hydroxide ions
what does pH stand for?
power of hydrogen/potential of hydrogen
what pH is neutral?
7
the lower the number, the higher the….
acidity
what is the body’s pH during life?
7.4
immediately after death, pH becomes…
acidic
several hours after death, pH becomes…
basic after proteins breakdown and nitrogen is freed
when do embalming chemicals function best?
at optimal pH range (7.3 - 7.5 for formaldehyde, 7.3 up to 9 for glutaraldehyde)
what are buffers?
chemicals that resist change to pH, composed of a weak acid and conjugate base that together resist change (ex. borate)
what is EDTA, and what does it stand for?
a buffer used to resist pH change, stands for ethylenediaminetetraacetic acid)
what are salts?
ionic substances that contain metallic and nonmetallic elements
what is normal salt?
all replaceable hydrogen of corresponding acid has been replaced with metal
what is acid salt?
only part of the replaceable hydrogen off the acid has been replaced by a metal
what is basic salt?
contains a metal and one or more replaceable hydroxide ions
what is ionization?
dissociating of a substance into ions (ex. salt dissolving in water) - enables transfer of electricity
what is electrolysis?
the passage of an electrical current through a solution
most embalming fluids are made up of what sort of chemicals?
organic chemicals
how much of a barrel of a crude oil is used to purposes other than fuel?
25%
what is fractional distillation?
process by which crude oil is refined - distilled, boiled, processed individually in fractions
what is oil?
paraffins and isoprenoids, aromatics, asphaltenes, and nitrogen, sulfur, oxygen (NSO)
in nature, most organic chemicals are…
covalent
T or F: organic chemicals have higher boiling and melting points than inorganic/ionic compounds, as well as having absolute water solubility
false, organic chemicals have lower boiling and melting points and are not usually water soluble
what are some functions of embalming fluid?
preserving, hydrating, perfuming, colorizing, water treatment, cell penetration
what is the most important bond in organic chemistry?
the carbon-carbon bond
how many bonds can carbon form?
four bonds (tetravalent)
what are functional groups?
structural features that allow us to classify compounds by reactivity, composed of a group of atoms that have characteristic chemical behaviors (ex. alcohols, phenols, aldehydes)
what is the purpose of alcohol?
solvent and preservation
what is the purpose of arene?
morphine backbone, fragrance
what is the purpose of phenol?
disinfectant, cauterant, bleaching
what is the purpose of aldehyde?
preservation, disinfectant
what is the purpose of ketone?
cleaning, solvent
what are the purposes of carboxylic acids?
anticoagulant, water conditioner
what is the purpose of ester?
fragrance
what is the purpose of amide?
protein linkage
what is amine the product of?
decomposition
when drawing chemical structures…
carbons are shown for emphasis but are otherwise assumed to be present, hydrogen-carbon bonds are omitted to avoid clutter, all other atoms are shown
all of the chemically significant functional groups are built on a foundation of…
carbon-carbon single, double and triple bonds
what are alkanes?
series of compounds that contain carbon and hydrogen atoms with single covalent bonds - moat commonly used as octane, also found in waxes, lubricants and asphalt
what is an isomer?
same molecular formula, different connectivity
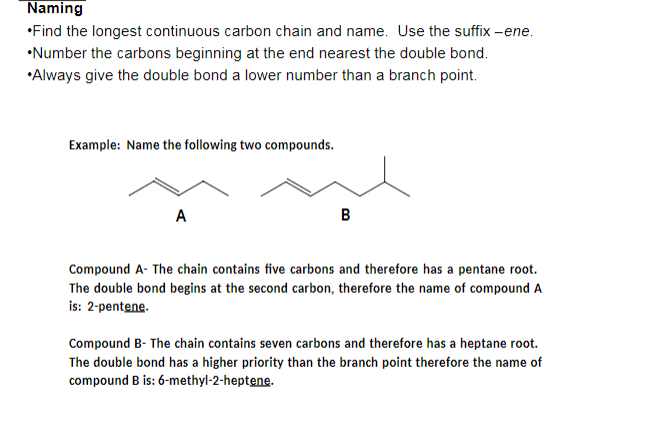
what are the three steps in naming compounds?
find the longest continuous carbon chain and use it as the central name
number the carbons such that the branch points have the lowest number possible
locate and name all alkyl groups attached to the longest
what are cycloalkanes?
alkanes in ring form, closed ring
what are alkenes?
hydrocarbons that contain a carbon-carbon double bond functional group, also known as ‘unsaturated’ bonds
are double bonds common in nature?
yes! important both biologically and in industrial chemicals
Naming Conventions