Titration Validation
1/20
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
21 Terms
What does anhydrous mean?
Containing no water.-
Contrast anhydrous to hydrated
Hydrated compounds contain water molecules.
Why is it desirable to use an anhydrous substance? (3 reasons)
Higher in purity.
Doesn’t allow for water to alter it’s mass.
Mass is accurate rep. of moles.
What is an Aliquot?
Known & accurate vol. delivered from pipette.
Describe when you would take an aliquot
Process of standardising solution during titration when you need to measure precise vol. of of solution your analysing.
Describe how you would take an aliquot
Use a volumetric pipette & take aliquot from primary standard to prepare secondary standard.
Why can chemicals never be returned to the storage bottle? (3 reasons)
Contamination risks.
Cross-contamination.
Loss of accuracy.
Why is it vital to always pour out minimum substance needed in any situation? (3 reasons)
Prevents waste.
Can be costly & environmentally harmful
Increases risk of spills, exposure, etc.
What is a primary standard?
Highly pure, stable substance with known conc. used to accurately determine conc. of unknown solution.
What form of prior treatment does NaCO3 require before use as a primary standard?
Drying (heating) to remove moisture & cooling in desiccator to prevent reabsorption of water.
Why should all solid be dissolved before transfer of solution from beaker to volumetric flask? (3 reasons)
Ensures accurate conc.
Prevents loss of solute.
Ensure homogenous solution
Is it necessary to weigh out exactly the calculated mass?
No as primary standard’s mass is true & accurate rep. of moles.
What are 3 key features of a primary standard?
Obtainable in pure form.
Shouldn’t alter during weighing.
Should have relatively high formula weight.
Why are the 3 key features important to ensure a reliable standard is produced?
Ensures molar conc. is accurate.
Ensures measurements are accurate.
Reduces weighing errors.
Why should a constant, minimum amount of indicator be used in titrations? (3 reasons)
Prevents indicator from affecting pH of solution.
Ensures clear & consistent endpoint.
Prevents overly intense or faint colour changes.
What indicator is best for strong acid and weak base titration?
Methyl orange as it changes colour in pH range of 3.1-4.4 & equivalence point of reaction is slightly acidic (around pH 4.5-5.5).
What should the following equipment be rinsed with and why?
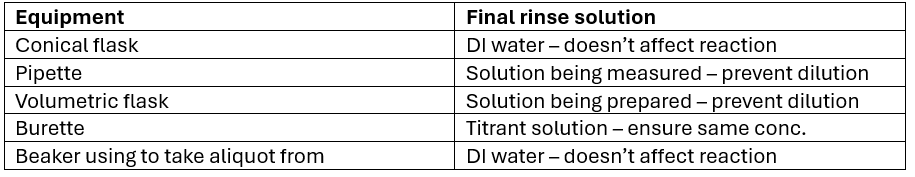
What is the impact if you rinsed with DI water instead of “solution to be used”?
Dilution errors affecting conc. of solution.
Can solution be blown out of pipette? (3 reasons)
Pipettes are calibrated to retain small residual volume.
Affects accuracy & precision.
Potential contamination.
Explain the difference between the end point and equivalence point?
Endpoint: point at which indicator changes colour visually signalling titration is nearly complete.
Equivalence point: theoretical point in titration occurring when exactly enough titrant has been added to completely neutralise acid or base.