Ionic Bonding in Edexcel IGCSE Chemistry
1/65
Earn XP
Description and Tags
chemistry
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
66 Terms
Ion
An electrically charged atom or group of atoms formed by the loss or gain of electrons.
Anion
Negative ions that form when atoms gain electrons, meaning they have more electrons than protons.
Cation
Positive ions that form when atoms lose electrons, meaning they have more protons than electrons.
Formation of Cations
All metals lose electrons to other atoms to become positively charged ions.
Formation of Anions
All non-metals gain electrons from other atoms to become negatively charged ions.
Charge of an Ion
The number of electrons that an atom gains or loses is the same as the charge.
Common Ions with Positive Charge
Includes Group 1 metals (1+), Group 2 metals (2+), Group 3 metals (3+), Silver (Ag), Copper(II) (Cu), Iron(II) (Fe), Iron(III) (Fe), Lead(II) (Pb), Zinc(II) (Zn), and Hydrogen (H).
Common Ions with Negative Charge
Includes Group 5 non-metals (3-), Group 6 non-metals (2-), Group 7 non-metals (1-), Hydroxide (OH), Carbonate (CO3 2-), Nitrate (NO3 -), and Sulfate (SO4 2-).
Outer Electron Shell
The electrons in the outermost shell of an atom that determine its ability to gain or lose electrons.
Compound Ions
Ions made from more than one element.
Group 1 Metals
Metals that typically form 1+ ions (e.g., Sodium - Na).
Group 2 Metals
Metals that typically form 2+ ions (e.g., Magnesium - Mg).
Group 3 Metals
Metals that typically form 3+ ions (e.g., Aluminum - Al).
Silver Ion
A common ion with a positive charge represented as Ag.
Copper(II) Ion
A common ion with a positive charge represented as Cu 2+.
Iron(II) Ion
A common ion with a positive charge represented as Fe 2+.
Iron(III) Ion
A common ion with a positive charge represented as Fe 3+.
Lead(II) Ion
A common ion with a positive charge represented as Pb 2+.
Zinc(II) Ion
A common ion with a positive charge represented as Zn 2+.
Hydrogen Ion
A common ion with a positive charge represented as H.
Ammonium Ion
A common ion with a positive charge represented as NH4+.
Group 5 Non-metals
Non-metals that typically form 3- ions (e.g., Nitrogen - N).
Group 6 Non-metals
Non-metals that typically form 2- ions (e.g., Oxygen - O).
Group 7 Non-metals
Non-metals that typically form 1- ions (e.g., Chlorine - Cl).
Ionic compounds
Typically have no overall charge.
Charge cancellation
The size of any positively charged ion is cancelled by the size of any negatively charged ion.
Iron(II) ion
Fe, which has a 2+ or +2 charge.
Sulfate ion
SO, which has a 2- or -2 charge.
Formula of iron(II) sulfate
FeSO4.
Swap-and-drop method
A method used when ions have different charges to determine the formula of an ionic compound.
Copper(II) ion
Cu, which has a 2+ or +2 charge.
Chloride ion
Cl, which has a 1- or -1 charge.
Formula of copper(II) chloride
CuCl2.
Worked example of iron chloride
The compound produced from iron wool and chlorine is FeCl3.
Complex ions
Ions such as carbonate, hydroxide, or sulfate that may require brackets in their formula.
Magnesium ion
Forms ions with a 2+ charge.
Hydroxide ion
Has a 1- charge.
Formula of magnesium hydroxide
Mg(OH)2.
Direct comparison method
A method to determine the formula of an ionic compound by comparing the charges of the ions.
Overall charge of ionic compounds
The overall charge is zero when the charges of the ions cancel each other out.
Number of chloride ions in FeCl3
Three chloride ions are needed to cancel the +3 charge on Fe.
Naming ionic compounds
The metal retains its name and the non-metal changes to its corresponding anion name.
Charge of iron ion in FeCl3
The iron ion has a 3+ or +3 charge.
Charge of chloride ion in FeCl3
The chloride ion has a 1- or -1 charge.
Mathematical representation of charge cancellation
Mathematically, (+3) + (-1) ≠ 0.
Ionic Bonds
Ionic bonds can be represented diagrammatically using dot-and-cross diagrams.
Dot-and-Cross Diagrams
The electrons from each atom should be represented by using solid dots and crosses.
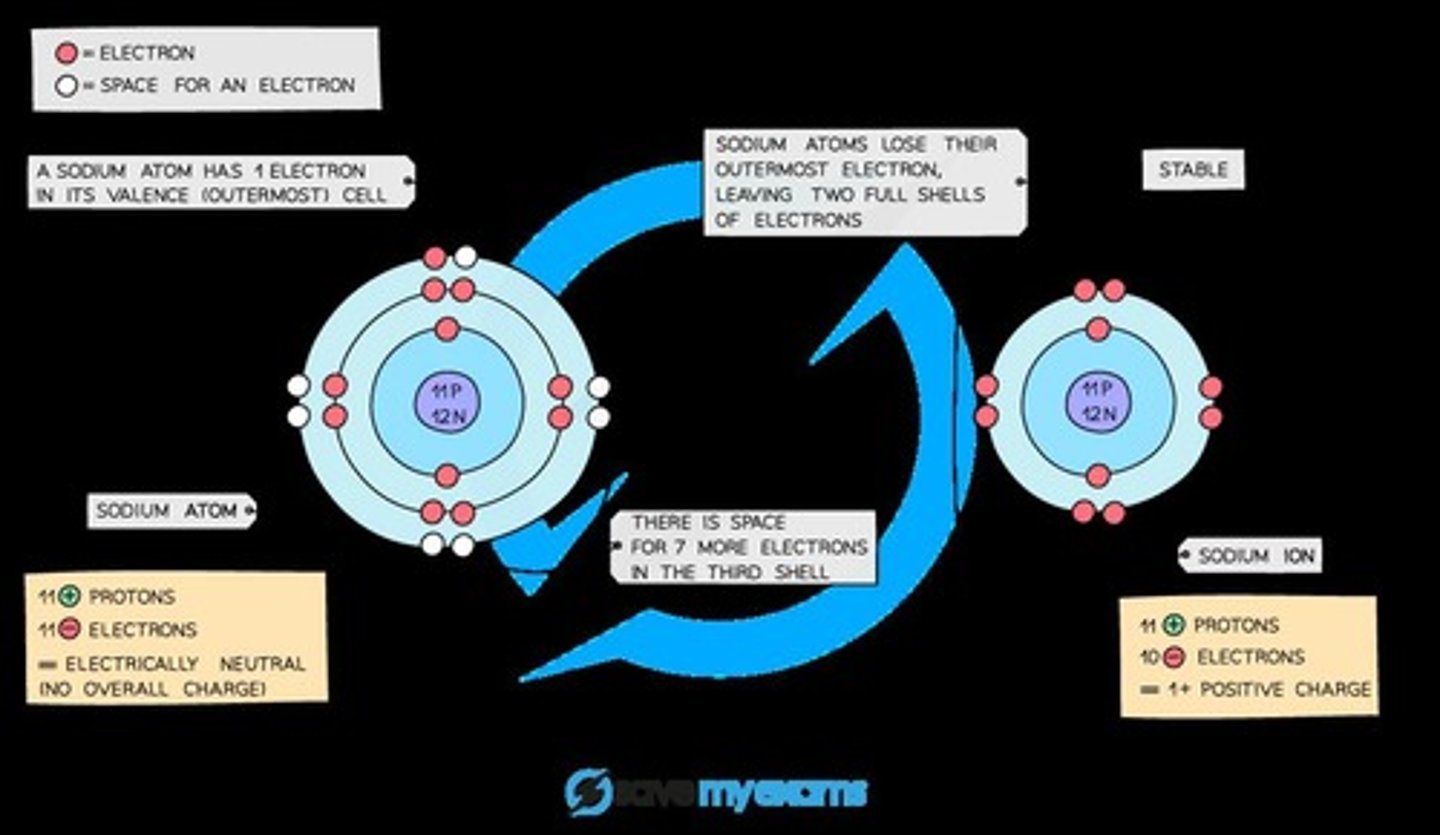
Sodium Ion
A positive sodium ion with the charge 1+ is formed when sodium loses one outer electron.
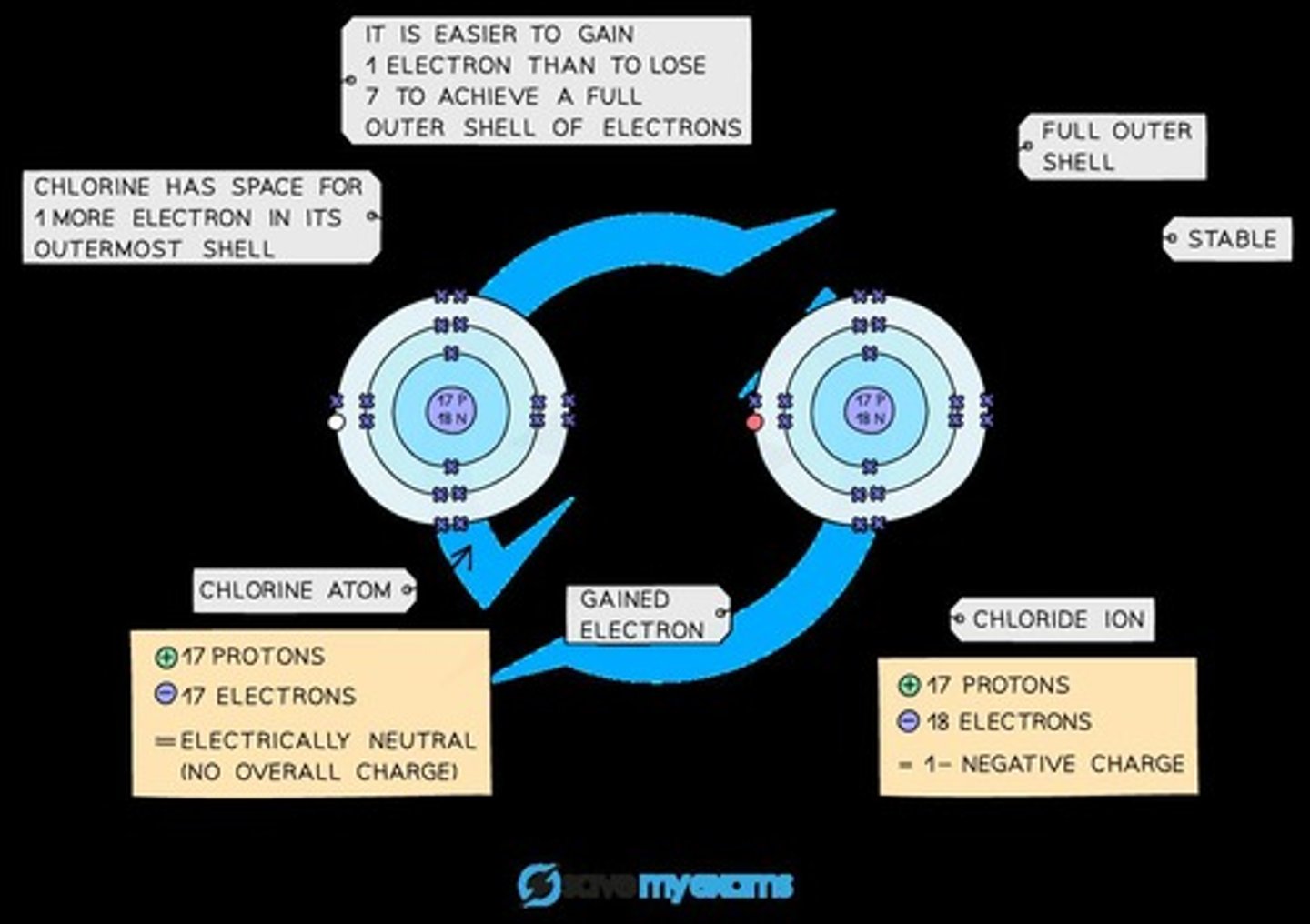
Chloride Ion
A chlorine atom will gain an electron to form a negatively charged chloride ion with a charge of 1-.
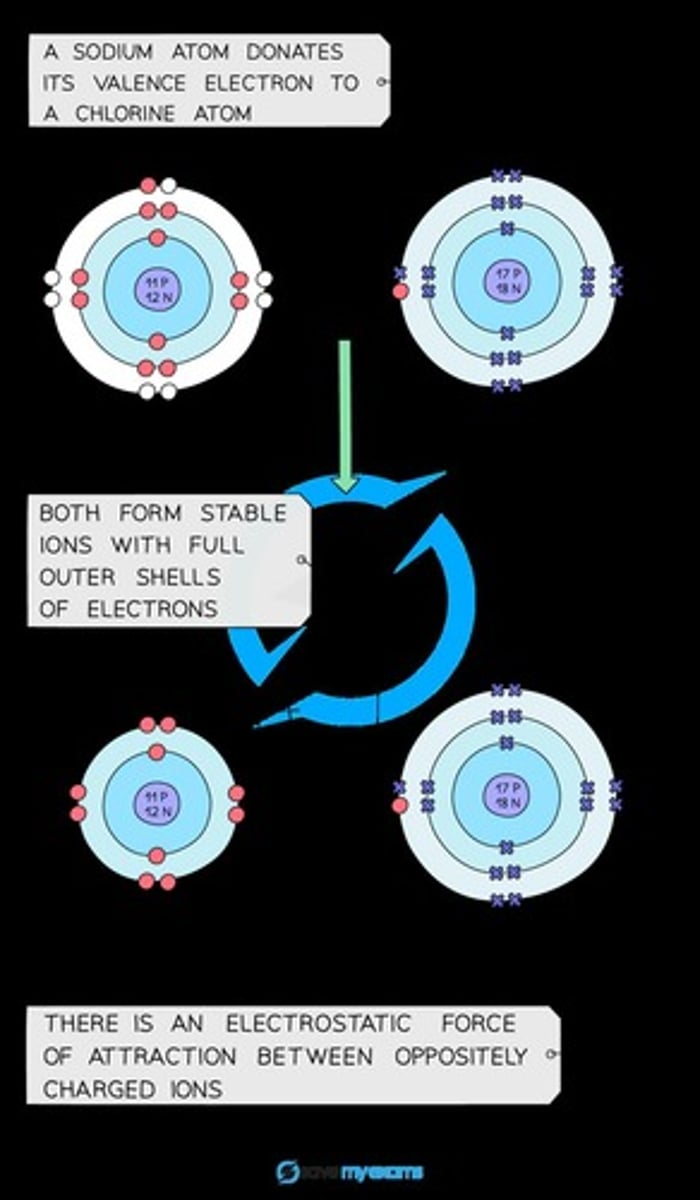
Sodium Chloride Formula
The formula of sodium chloride is NaCl.
Magnesium Ion
A positive ion with the charge 2+ is formed when magnesium loses two outer electrons.
Oxygen Ion
Oxygen atom will gain two electrons to form a negative ion with charge 2-.
Magnesium Oxide Formula
The formula of magnesium oxide is MgO.
Ionic Compounds
An ionic compound consists of a regular arrangement of alternating positive and negative ions.
Electrostatic Forces
Strong electrostatic forces of attraction exist between the oppositely charged ions.
Giant Ionic Lattice
Thousands of positive and negative ions in an ionic compound form a giant lattice structure.
Melting Points of Ionic Compounds
Compounds with giant ionic lattices have high melting points.
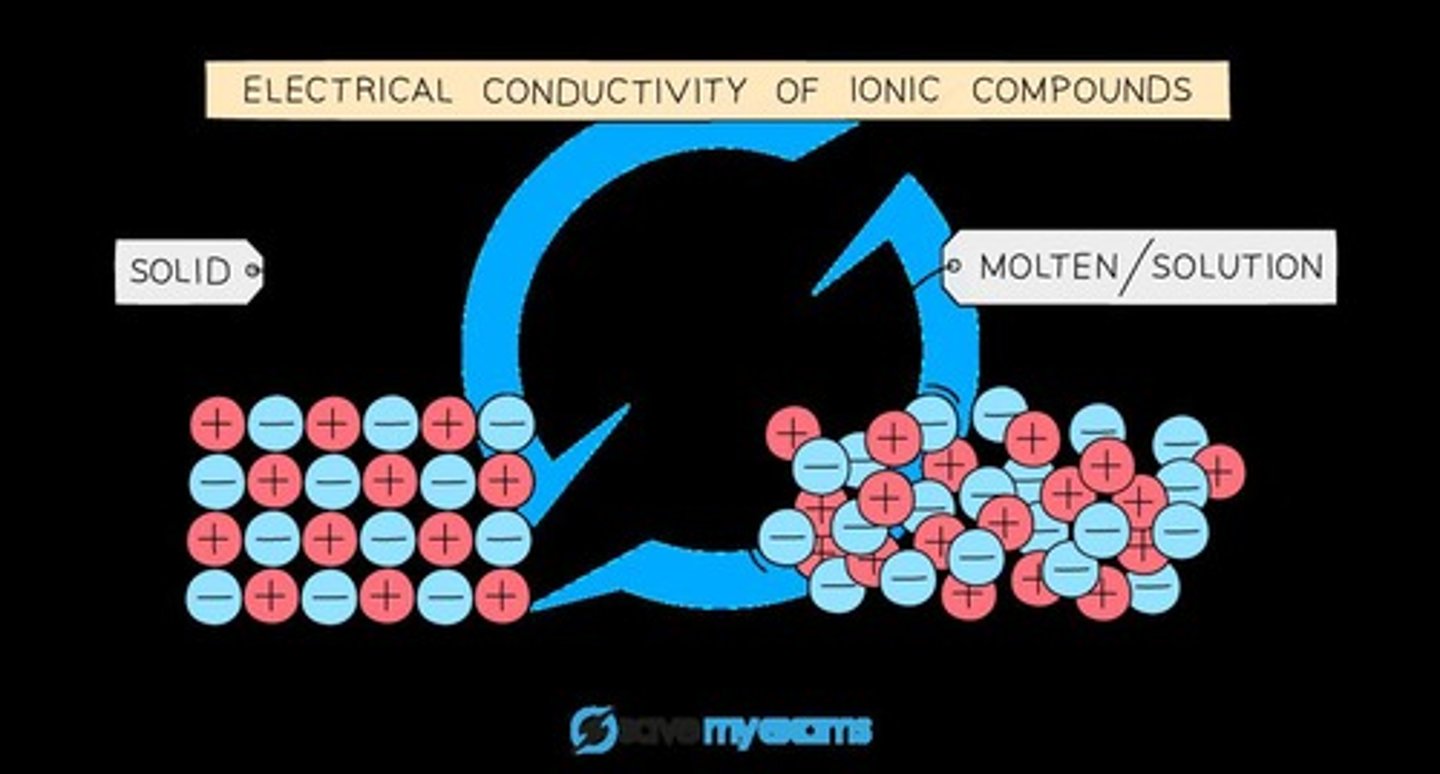
Conductivity in Solid State
Ionic compounds are poor conductors in the solid state because the ions are in fixed positions.
Conductivity in Molten State
Ionic compounds are good conductors of electricity in the molten state or in solution.
Energy to Overcome Forces
The strong electrostatic forces need lots of energy to overcome them.
Higher Melting Point
The greater the charge on the ions, the stronger the electrostatic forces and the higher the melting point will be.
Common Mistake in Exams
A common mistake is to say that ionic compounds conduct electricity because 'electrons' move, when it should be the ions that can move and carry a charge.
Fixed Positions of Ions
In the solid state, the ions are unable to move and carry a charge.
Movement of Particles
Molten or aqueous particles move and conduct electricity but cannot in the solid state.
Exam Drawing Requirement
For exam purposes, you need only show the outer electrons in dot & cross diagrams.
Groups for Dot & Cross Diagrams
You should be able to draw dot & cross diagrams for combinations of ions from groups 1, 2, 3, 5, 6, and 7.