C5 - Energy Changes
1/14
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
15 Terms
Why do all chemical reactions involve energy changes?
Because energy is needed to break bonds and is released when bonds are made
Exothermic if the reaction gets…
Hot
Endothermic if the reaction gets…
Cold
Energy changes practical
React an acid with an alkali, and measure the max. temp reached. Repeat with increasing volumes of alkali
The max. temp will eventually decrease as the alkali is in excess (same energy released but larger volume)
Plot graph and where two lines of best fit meet is the volume needed to neutralise

1?
Activation energy
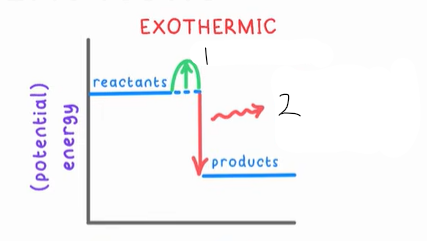
2?
Net energy out, increases temp, then lost to surroundings
In exothermic reactions, why must there be a net decrease in potential energy?
So there can be a net increase in kinetic energy (increase in temp)

Activation energy (from surroundings)
In endothermic reactions, why must there be an increase in potential energy?
So there is a decrease in kinetic energy (temp. decreases)
Bond energy
The amount of energy required to break bonds, the same as the amount of energy released when a bond is formed
Bond energy for C-H
413 kJ/mol
Bond energy for O=O
495 kJ/mol
Bond energy for C=O
799 kJ/mol
Bond energy for O-H
467 kJ/mol
How to work out bond energies for a reaction
Write out equation
Draw structure to see bonds
Calculate energy (number of bonds x bond energy) for each molecule
If energy before is lower, reaction is exothermic. If energy before is higher, reaction is endothermic
Net potential energy change = start energy - end energy