Impregnation / Infiltration, & Embedding and Trimming of Tissue Block- MIDTERMS L10
1/51
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
52 Terms
IMPREGNATION
Also known as INFILTRATION
Process whereby the clearing agent is completely removed from the tissue & replaced by a medium that will completely fill all the tissue cavities, thereby giving a firm consistency to the specimen
Process of replacing the clearing agent with the infiltrating medium.
The medium used to infiltrate the tissue is usually the same medium used for embedding
FOUR TYPES OF IMPREGNATION AND EMBEDDING MEDIA
Paraffin wax Impregnation
Celloidin (Collodion) Impregnation
Gelatin Impregnation
Plastic
BUTSCHLII
The man who introduced paraffin wax embedding
PARAFFIN
Simplest, most common and the BEST infiltrating/ embedding medium.
Process is very rapid, allowing sections to be prepared within 24 hrs
PARAFFIN DISADVANTAGE
Overheated paraffin makes the specimen brittle
Prolonging will cause excessive tissue shrinkage and hardening
NOT recommended for fatty tissues (the dehydrants and clearing agents used in the process dissolve and remove fat from the tissues).
AFTER CLEARING
Tissue is submerged in 2 or more changes of melted paraffin wax.
TEMPERATURE OR PARAFFIN OVEN
55-60*C
(Paraffin oven must be maintained at a temperature 2-5*C above the MP of the paraffin wax to be used.)
MELTING POINT OF COMMON WAXES
45, 52, 56, 58°C
WAX WITH MELTING POINT OF 56*C
Is normally used for routine work.
LAB TEMPERATURE OF 20-24*C
Paraffin wax melting point is 54-58*C
LAB TEMPERATURE OF 15-18*C
Melting point of wax 50*C to 54*C
HARD TISSUE
Require wax with higher melting point
WHEN WAX HAS BEEN REUSED
Some water is mixed with it
IF EXCESSIVE WATER ACCUMULATES
This may impair the impregnating capacity of the medium.
TO REMOVE EXCESS WATER
Heat the wax to 100-105*C
(Paraffin wax may be used twice only!)
THREE WAYS BY WHICH PARAFFIN WAX IMPREGNATION AND EMBEDDING MAY BE PERFORMED
Manual Processing
Automatic Processing
Vacuum embedding
MANUAL PROCESSING
AUTOMATIC PROCESSING
With the use of autotechnicon
AUTOTECHNICON
Fixes, dehydrates, clears and infiltrates tissues
For rapid diagnosis with less technicality
2-3 changes of wax is required
VACUUM EMBEDDING
Involves the wax impregnation under negative atmospheric pressure (400-500 mmHg) inside an embedding oven.
Gives the fastest results
To hasten removal of air bubbles and clearing agent
Promote rapid wax penetration
Time reduce from 25-75% of the normal time require
FACTORS AFFECTING PARAFFIN WAX IMPREGNATION
Nature and size of the tissues to be processed
Type of clearing agents to be used
PRECAUTIONS OBSERVED IN PARAFFIN WAX IMPREGNATION
Tissue should not be left for longer periods of time
Maintained a temperature 2 to 5°C above the melting point
Paraffin wax must be pure
Fresh wax should be filtered before use
When using automatic tissue processing machine, wax usually becomes admixed with the clearing agent, especially in the first beaker
For fixed knife microtomes, a relatively hard wax with a higher melting point is recommended
SUBSTITUTES FOR PARAFFIN WAX
Paraplast
Ester Wax
Water Soluble waxes
PARAPLAST
Mixture of highly purified paraffin and synthetic plastic polymers
MELTING POINT OF PARAPLAST
56-57*C
TYPES OF PARAPLAST
Embeddol
Bioloid
Tissue mat
EMBEDDOL
Melting point 56-58°C
BIOLOID
Semisynthetic recommended for embedding eye
TISSUE MAT
Product of paraffin
ESTER WAX
Has lower melting point 46-48°C
Harder than paraffin
Not soluble in water but soluble in 95% Ethyl Alcohol
WATER SOLUBLE WAXES
Mostly polyethylene glycols
MELTING POINT: 38-42°C or 45-56°C
CARBOWAX
Most common water-soluble wax (hydroscopic)
4 changes of carbowax for routine processing
70%,90%,100% (2x)
56°C at 30 minutes
45 minutes and 1 hour (with agitation)
CELLOIDIN (COLLODION) [CELLOIDIN IMPREGNATION]
Purified form of nitrocellulose soluble in many solvents
Suitable for specimen with hollow cavities
Recommended for processing neurological tissues
Avoiding the crumbling of tissues during sectioning
Does not require heat
DISADVANTAGE OF CELLOIDIN IMPREGNATION
Very slow (lasting for several days or weeks)
Very thin section difficult to cut
Photomicrographs are difficult to obtain
Very volatile
TWO METHODS FOR CELLOIDIN IMPREGNATION
Wet Celloidin
Dry Celloidin
WET CELLOIDIN
Recommended for bones,teeth, large brain sections and whole organs.
DRY CELLOIDIN
Preferred for processing of whole eye sections
NITROCELLULOSE METHOD [CELLOIDIN IMPREGNATION]
Low Viscosity Nitrocellulose (L.V.N.)
LOW VISCOCITY NITROCELLULOSE (L.V.N.)
Is another form of celloidin soluble in equal concentration of ether and alcohol, with lower viscosity, allowing it to be used in higher concentration and still penetrate tissue rapidly
It forms a harder tissue block and makes cutting of thinner sections possible
The tendency to tissues to crack may be prevented by adding plasticizers (oleum ricini or castor oil) when embedding chrome-mordanted tissues.
L.V.N. is more explosive than celloidin.
GELATIN IMPREGNATION
Rarely used except when dehydration is to be avoided and when tissue are subjected to histochemical and enzyme studies
Used as an embedding medium for delicate specimens and frozen tissue sections.
It is water soluble, does not require dehydration and clearing, although fixatives should still washed out by running water.
Tissue is placed in 10% gelatin with 1% phenol for 24 hours, transferred to 20% gelatin with 1% phenol for the next 12 hours, finally to another fresh solution of 20% gelatin with 1% phenol which is then allowed to cool in a refrigerator until impregnation and embedding are completed.
Tissue should not be more than 23 mm. thick
The volume of the impregnating medium should be at least 25x the volume of the tissues.
PLASTIC (RESIN)
It has provided superior results for light microscopic studies, particularly in hard tissues
Tissue sections must be 4-6um, such in renal biopsies and bone marrow biopsies.
Plastic are classified into epoxy, polyester, or acrylic, based on their chemical composition
EMBEDDING
Process by which the impregnated tissue is placed into a precisely arranged position in a mold containing a medium which is then allowed to solidify.
ORIENTATION
Process by which the tissue is arrange in precise position in the mold during embedding, on the microtome before cutting and on the slide before staining
OTHER EMBEDDING METHODS
Celloidin or Nitrocellulose method
Double Embedding Method
CELLOIDIN OR NITROCELLULOSE METHOD
Recommended for embedding hard tissues
DOUBLE EMBEDDING METHOD
Process in which tissues are first infiltrated with celloidin and subsequently embedded in paraffin mass.
Used to facilitate cutting of large block of dense firm tissue like the brain.
TYPES OF BLOCKING- OUT EMBEDDING MOLDS
Leuckhart’s embedding mold
Compound embedding Unit
Plastic embedding rings & base molds
Disposable embedding molds
Peel-away
Plastic Ice trays
Paper boats
LEUCKHART’S EMBEDDING MOLD
Consist of two L-shaped strips of heavy brass of metal arranged on a flat metal plate and which can be moved to adjust the size of the mold to the size of the specimen
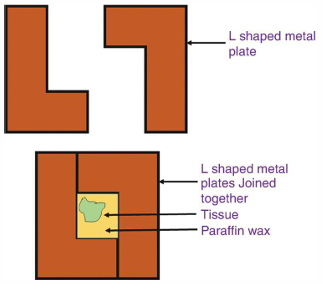
COMPOUND EMBEDDING UNIT
Made up of a series of interlocking plates resting on a flat metal base, forming several compartments
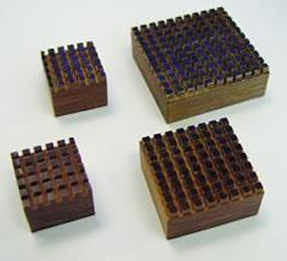
PLASTIC EMBEDDING RINGS AND BASE MOLDS
Consist of a special stainless steel base mold fitted with a plastic embedding ring, which later serves as the block holder during cutting.

TRIMMING OF SECTIONS
Is the process of removing the excess wax by cutting off from the block to expose the tissue surface in preparation for sectioning.
ONCE THE WAX HAS SOLIDIFIED
The wax block is removed from the mold
The identification number is noted & the excess wax is cut off from the block to expose the tissue surface in preparation for cutting