Silverstein and Hopper Chapter 56: Potassium Disorders
1/36
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
37 Terms
What is the most abdundant intracellular anion?
Potassium
What % of potassium is located in the intracellular compartment?
98-99%
What are the feedback mechanisms involved in potassium regulation?
pH regulation
Changes in osmolality
Hormones including insulin, catecholamines, and aldosterone
How does the body maintain pH in metabolic alkalosis?
In metabolic alkalosis, the body maintains pH by causing more potassium to move intracellularly in exchange for cellular H+ ions
Hyperosmolality causes the translocation of water from the cellular space, which drags cellular potassium into the extracellular fluid space
What substances transfer potassium from the extracellular space to the intracellular space?
Insulin, catecholamines, and aldosterone
Any increase in extracellular fluid potassium concentration triggers aldosterone release
Aldosterone acts at the distal renal tubules to increase Na+/K+ ATPase activity -> promotes the transluminal transfer of potassium ions through the collecting duct principal cells into the renal tubular lumen, allowing for potassium excretion and sodium reabsorption
Kaliuretic Feedforward Control
Potassium control mechanism that responds to signals in the external environment and involves sensors in the stomach and the hepatic portal regions
Sensors detect local changes in potassium concentrations resulting from potassium ingestion and signal the kidney to alter potassium excretion to restore potassium balance
One without the influence of aldosterone
How does the body counteract hypokalemia?
Transfer of potassium from the intracellular space into the extracellular space
Hypokalemia
Occurs when the serum potassium concentration is less than 3.5 mEq/L
General Causes of Hypokalemia
Disorders of internal balance
Disorders of external balance
Causes of Hypokalemia - Disorders of Internal Balance (Redistribution)
Metabolic alkalosis
Insulin administration
Increased levels of catecholamines
B-adrenergic agonist therapy or intoxication
Refeeding syndrome
Causes of Hypokalemia - Disorders of External Balance (Depletion)
Renal potassium wasting
Prolonged inadequate intake
Diuretic drugs
Osmotic or postobstructive diuresis
Chronic liver failure
Inadequate parenteral fluid supplementation
Aldosterone-secreting tumor or any cause of hyperaldosteronism
Prolonged vomiting associated with pyloric outflow obstruction
Diabetic ketoacidosis
Renal tubular acidosis
Severe diarrhea
Ingestion of barium-containing party sparklers
Glucocorticoid drugs
Metabolic Consequences of Hypokalemia
Glucose intolerance
Neuromuscular Consequences of Hypokalemia
Potassium necessary for maintenance of normal resting membrane potential
Skeletal muscle weakness from hyperpolarized (less excitable) myocyte plasma membranes that may progress to hypopolarized membranes
Ventroflexion of the head and neck, a stiff stilted gaint, and a plantigrade stance may be evident
Rhabdomyolysis which can have a toxic effect on renal tubules
Smooth muscle impairment can occur and predispose to paralytic ileus and gastric atony
Neuromuscular signs seldom present until potassium levels fall below 2.5 mEq/L
Cardiovascular Consequences of Hypokalemia
In the myocardial cell, a high intracellular/extracellular potassium concentration ratio induces a state of electrical hyperpolarization leading to prolongation of the action potential
May predispose the patient to atrial and ventricular tachyarrhythimas, atrioventricular dissociation, and ventricular fibrillation
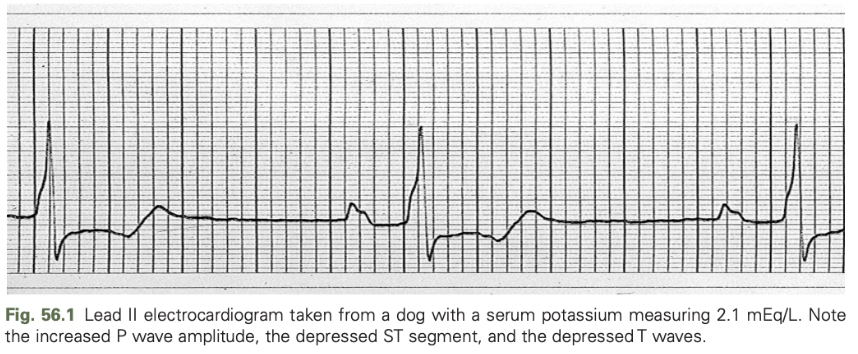
ECG findings in hypokalemia are less reliable than hyperkalemia
Include depression of the ST segment and prolongation of the QT interval
Increased P wave amplitude, prolongation of the PR interval, and widening of the QRS complex may also occur
Hypokalemia predisposes to digitalis-induced cardiac arrhythmias and causes the myocardium to become refractory to the effects of class I antiarrhythmic agents
Main Management Objectives of Hypokalemia
Deterring continued potassium losses
Replacing potassium deficits while considering the preparation type and route of administration
Correcting the primary disease process
Treating Hypokalemia Associated with Metabolic Alkalosis
Normalizing blood pH
Replacing the potassium deficit
Correcting the cause of the alkalosis
Treating Hypokalemia Associated with Primary Hyperaldosteronism
Removing the cause of the excess aldosterone and/or counteracting the hormone's effect at the distal renal tubule by treating the patient with an aldosterone antagonist such as spironolactone
Treating Moderate to Severe Hypokalemia in the Anoretic or Vomiting Patient
Parenteral administration of potassium chloride solution (or potassium phosphate in hypophosphatemic patients)
Rate of potassium infusion should seldom exceed 0.5 mEq/kg/hr for treatment of patients with mild to moderate hypokalemia
In profoundly hypokalemic patients (serum potassium <2.5 mEq/L) with normal or increased urine output, the rate can be increased to 1-1.5 mEq/kg/hr with ECG monitoring
Conditions that May Predispose an Animal to Adverse Effects of a Potassium Infusion
Oliguria
Anuria
Hypoaldosteronism (Addison's disease)
Coadministration of potassium-sparing drugs (spironolactone, triamterene)
Exception to the “Safe” Rate of Administration of Potassium
When marked hypokalemia causes apnea, under which circumstances an intravenous bolus of 0.01 ml/kg of a 2 mEq/ml solution can be live saving
Anticipated Complications of Treatment of Hypokalemia
Hyperkalemia can occur from excessive potassium supplementation
Hypokalemic neuromuscular dysfunction is worsened and refractoriness to therapy may be evident when metabolic alkalosis, hypomagnesemia, and hypocalcemia coexist
All acid-base disorders and electrolyte deficiencies must be corrected to attain normal neuromuscular function
Hyperkalemia
Occurs when the serum potassium concentration exceeds 5.5 mEq/L and is considered life threatening at serum concentrations greater than 7.5 mEq/L
What are the four basic disturbances that result in hyperkalemia?
Increased intake or administration
Translocation from the intracellular to extracellular fluid space
Decreased renal excretion
Artifactual or pseudohyperkalemia
Causes of Hyperkalemia - Increased Intake or Administration
To avoid life-threatening neuromuscular side effects, the IV rate of potassium should not exceed 0.5 mEq/kg/hr
Administration of packed red blood cells that are past the expiration date
Certain medications such as ACE inhibitors, angiotensin receptor blockers, potassium-sparing diuretics (e.g. spironolactone), or nonselective B-blocking drugs (e.g. propranolol), heparin, cyclosporine, and tacrolimus
Causes of Hyperkalemia - Translocation from the Intracellular to Extracellular Fluid Space
Mineral acidosis (respiratory acidosis, uremia, or pharmacologic induction by ammonium chloride, hydrogen chloride, or calcium chloride infusions) causing potassium to move out of the intracellular space in exchange for hydrogen ions
Organic acids such as lactate and ketoacids rarely cause this effect because of their ability to maintain electroneutrality across the cell membrane
Heat stroke
Crushing injuries
Tumor lysis syndrome associated with chemotherapy
After radiation therapy in dogs with lymphosarcoma
Cats treated with thrombolytic agents for aortic thromboemolism as a result of reperfusion of the affected limbs
Occurs commonly during cardiopulmonary resuscitation and immediately following the return of spontaneous circulation due to ischemia induced cellular damage and release of large amounts of intracellular potassium
Diabetic patients
Insulin deficiency that results in decreased cellular uptake of potassium
Hyperosmolality that potentiates potassium translocation with water due to "solute drag" effect
Decreased potassium excretion related to renal dysfunction
Insulin therapy normalizes the serum potassium concentration by correcting the insulin deficiency and hyperoasmolality, enabling relocation of potassium to the intracellular space, and decreasing the need for further protein catabolism
Causes of Hyperkalemia
In animals with chronic renal disease, adaptation in the kidneys promotes an increase in fractional potassium excretion as well as adaptations in the GI system with increased fecal excretion
Distal tubule is dependent on adequate glomerular filtration rate and urine flow to excrete potassium
Until effective urine output and improvement of GFR return, any reduction of potassium levels can only occur with therapies such as hemodialysis or peritoneal dialysis
Hyperkalemia may be due to dietary potassium exceeding renal excretion as well as ACE inhibitor therapy that may be used to treat hypertension and proteinuria
Feeding a potassium reduced diet can resolve hyperkalemia in these animals
Other drug therapies such as nonspecific B-blockers, cardiac glycosides, ACE inhibitors, angiotensin receptor blockers, cyclosporine, tacrolimus heparin, and trimethoprim may also contribute to hyperkalemia in these patients with diminished renal function
Patients with classic, severe hypoadrenocorticism typically have hyperkalemia and hyponatremia and a sodium:potassium ratio less than 27:1
An ACTH stimulation test is essential to differentiate this disease from AKI because these patients might also be azotemic and have resting serum cortisol values <1.0 ug/dL
In the absence of aldosterone, the resulting natriuresis causes a reduced effective circulating volume, which further impairs distal tubule potassium excretion
Decreased volume also leads to reduced renal perfusion, prerenal azotemia, and further potassium retention
Initial therapy should include restoration of the effective circulating volume
ECG Changes in Patients with Hyperkalemia
Peaked, narrow T waves
Prolonged QRS complex and interval
Depressed ST segment
Depressed P wave
Atrial standstill
Ventricular flutter/fibrillation
Balanced Electrolyte Solutions vs NaCl for Hyperkalemia
Balanced electrolyte solutions that contain potassium can be used to stabilize these patients
A study found no difference in rate to normalization of potassium between a balanced electrolyte IV fluid containing potassium and 0.9% NaCl, however the balanced solutions led to a more rapid correction in acid base status
Pseudohyperkalemia
Artifactual increase in potassium from potassium released from increased numbers of circulating blood cells, especially platelets and leukocytes
Typically occurs with significantly elevated counts (>1,000,000 platelets and >100,000 leukocytes)
Confirmation can be made by determining the plasma potassium concentration because this should not be affected by changes in platelet or white blood cell numbers
Consequences of Hyperkalemia
Hyperkalemia results in changes in the cell membrane excitability, causing changes in cardiac myocyte excitation and conduction
Muscle weakness can occur when the serum potassium concentration exceeds 7.5 mEq/L
Ratio of intracellular to extracellular potassium is the main factor in determining the cardiac resting membrane potential
In hyperkalemic patients, the concentration gradient across the cardiac cell membranes is reduced, leading to a less negative resting membrane potential
Makes these cardiac cell membranes more excitable
Elevated potassium also inactivates some of the sodium-potassium channels during the resting phase, making these cells slower to reach threshold potential
An overall decrease in potassium permeability means that efflux of potassium in repolarization is delayed, slowing the cell's recovery
Acidemia results in extracellular shift in potassium as well as decreasing the B-adrenergic receptors in cardiac tissues
Atrial standstill, ventricular flutter, and asystole are reported effects
Sinus tachycardia, third degree heart block, ventricular premature complexes, and atrioventricular dissociation have also been reported
Treatment of Hyperkalemia
ECG and blood pressure monitoring recommended
Exogenous potassium supplementation discontinued
Evaluate for urinary tract abnormalities (uroabdomen, ureteral obstruction, urinary stones)
In asymptomatic animals with normal urine output, serum potassium concentrations between 5.5 and 6.5 mEq/L rarely require immediate therapy
Replacement fluids can be used to rehydrate the patient and correct for prerenal azotemia and promote diuresis
Loop diuretics or thiazide diuretics can increase urinary potassium excretion, but use must follow rehydration
Treatment of Hyperkalemia in Patients with Potassium >7.5 mEq/L
10% calcium gluconate or calcium chloride can be administered to antagonize the cardiotoxic effects of hyperkalemia, but this has no effect on serum potassium concentration
Calcium functions by increasing the threshold potential to maintain the gradient between that and the resting membrane potentials
Reduces membrane excitability
Give slowly over 15-20 minutes with ECG monitoring
B-adrenergic agonists (terbutaline, albuterol, epinephrine), sodium bicarbonate, and dextrose with or without insulin can be administered to reduce serum potassium concentrations
Shift potassium intracellularly, lowering serum potassium
Use caution with sodium bicarbonate because of need for slow administration and potential to cause severe alkalosis and paradoxical cerebral acidosis
Rarely used
Calcium gluconate, dextrose/insulin, and B-adrenergic agonists are first-line therapies for management of hyperkalemia treatment
How do you reduce total body potassium in the oliguric or anuric patient?
Extracorporeal therapy
MOA of 10% Calcium Gluconate in Hyperkalemia
Increases threshold voltage but will not lower serum potassium
MOA of Sodium Bicarbonate in Hyperkalemia
Causes metabolic alkalosis allowing for potassium to move intracellularly, paradoxical CNS acidosis with rapid administration
MOA of 50% Dextrose in Hyperkalemia
Allows for translocation of potassium into the intracellular space in the presence of endogenous insulin
Add exogenous insulin if needed
MOA of Terbutaline for Hyperkalemia
Stimulates Na+/K+ to cause translocation of potassium into the cell