Chemistry AQA GCSE Rates of Reaction
1/32
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
33 Terms
How would you calculate rate of a reaction? Give two equations.
Mean rate of reaction = quantity of reactant used / time taken
Mean rate of reaction = quantity of product formed / time taken
Give three units for the quantity of reactant used/product formed - for each, write the word, symbol and what it represents
Grams (g) - mass
Centimetres cubed (cm^3) - volume
Moles (mol) - amount of particles
Give three units for rate of reaction - for each, state the word and symbol
Grams per second (g/s)
Centimetres cubed per second (cm^3/s)
Moles per seconds (mol/s)
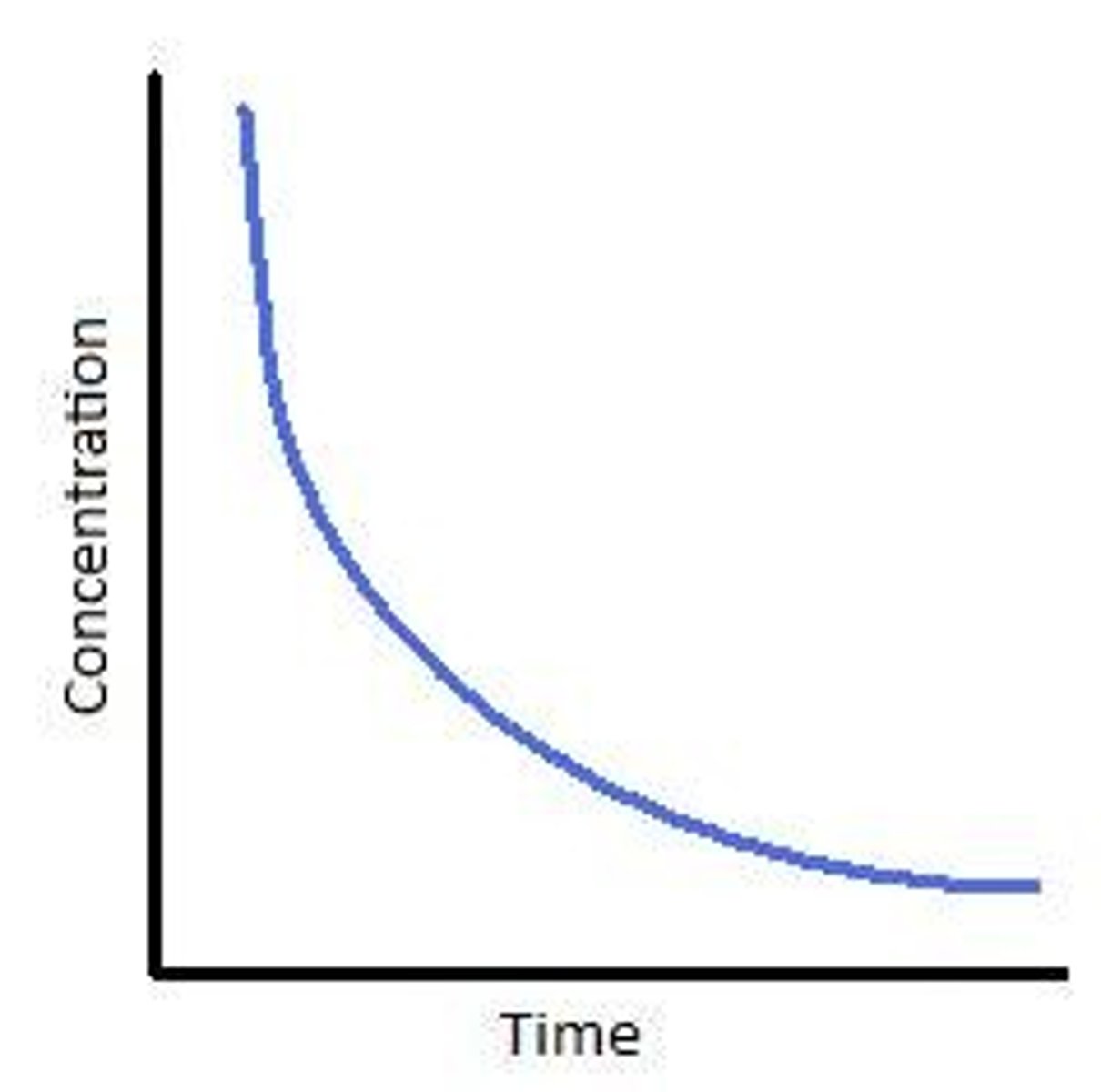
Draw a graph showing the quantity of reactant used against time - state what this suggests about rate of reaction
This suggests that as time increases rate of reaction decreases over time
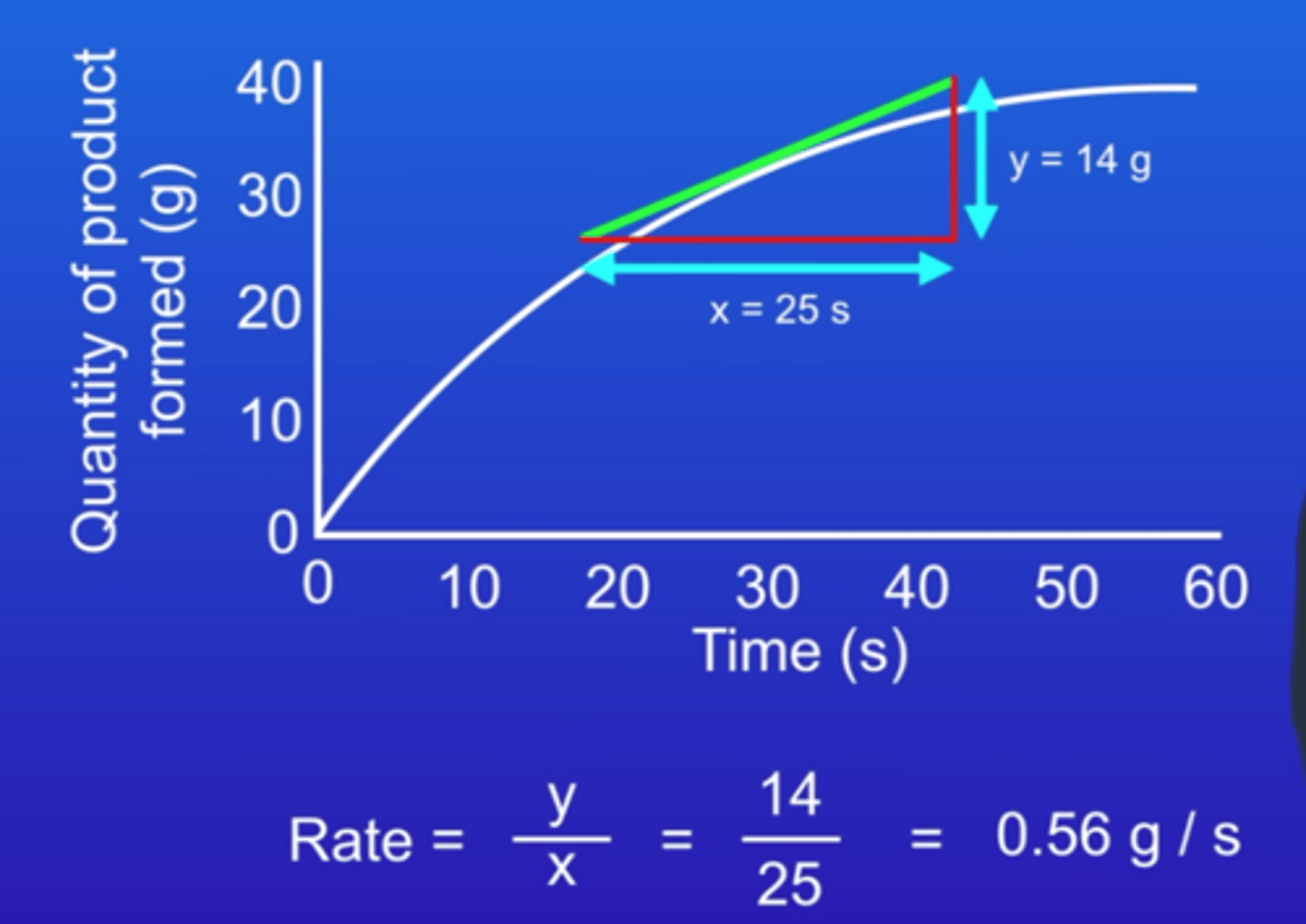
Draw a graph showing the quantity of product formed against time - how would you calculate rate of reaction at a specific point?
Draw a tangent at the point of graph you want to find the rate of reaction to - extend this tangent until it hits an axis or the origin. Then, calculate the change in y and the change in x. Change in y/change in x is the gradient of the tangent, which is the rate of reaction.
Name 5 factors which affect the rates of chemical reactions.
•Concentration of reactants in solution
•Pressure of reacting gases
•Surface Area of solid reactants
•Temperature
•Presence of catalysts
Describe a method to investigate how changes in concentration affect the rates of reactions involving a change in colour/turbidity
First, use a measuring cylinder to put e.g. 10cm^3 of 0.2 mol/dm^3 sodium thiosulphate solution into a conical flask. Place a black cross on to a white tile, and place the conical flask over the black cross. Next, add the same amount of 0.2 mol/dm^3 hydrochloric acid into the conical flask using a measuring cylinder. Swirl the solution and start a stopwatch. Looking from the top of the flask, stop the stopwatch when the cross can no longer be seen. Repeat for other concentrations of sodium thiosulphate solution. Repeat the whole investigation and take a mean result.
Describe a method to investigate how changes in concentration affect the rates of reactions involving measuring a volume of gas produced.
First, use a measuring cylinder to place e.g. 50cm^3 of 0.2mol/dm^3 e.g. hydrochloric acid (it must be an acid) into a conical flask. Then, drop a ribbon of e.g. magnesium (must be a metal more reactive than hydrogen) and quickly attach the conical flask to a bung and a gas syringe. Start a stopwatch. Every ten seconds, record the amount of hydrogen gas in the gas syringe until no more hydrogen is produced. Repeat for different concentrations of the acid. Repeat the whole investigation and take a mean.
For the Rates required practical, state one source of systematic error when recording a change in turbidity and state how this can be reduced
Different people have different eyesights, so different people willsee the cross disappear faster than others - to reduce this, you can either:
•Repeat the experiment many times with many different people
or
•Have many people looking into the flask and stop the stopwatch once half the people agree it is cloudy.
What is the purpose of collision theory?
To explain how various factors affect rates of reactions
State collision theory
Chemical reactions can occur only when reacting particles collide with each other and with sufficient energy.
Define activation energy
The minimum amount of energy that particles must have to react
Explain how increasing the concentration of a reactant affects rate of reaction.
This will increase the amount of particles in a fixed volume, increasing the frequency of collisions and increasing the rate of reaction.
Explain how decreasing the pressure of a reactant affects the rate of reaction.
This will decrease the amount of particles in a fixed volume, decreasing the frequency of collisions and decreasing the rate of reaction.
Explain how increasing the surface area of a reactant affects the rate of reaction.
This will increase the amount of particles which can be exposed to reactants, increasing the frequency of collisions and increasing the rate if reaction.
Explain how increasing the temperature of a reacting mixture affects the rate of reaction
This will increase the kinetic energy of the particles in the mixture, increasing the frequency of collisions and making the collisions more energetic, increasing the rate of reaction
Explain how making pieces of a reactant bigger affects the rate of reaction.
This will decrease the surface area to volume ratio of the substance, decreasing the amount of reactant particles which are exposed to the surface, decreasing the frequency of collisions, decreasing the rate of reaction
State the relationship between frequency of collisions and rate of reaction.
Directly proportional.
Define catalyst and explain how they affect rate of reaction.
Substances which increase the rate of reaction by providing a different pathway for the reaction that has a lower activation energy without being used up during the reaction.
Briefly explain why no one substance is used as a "universal catalyst".
Different reactions need different catalysts
Briefly define enzyme
Catalysts in biological systems
Draw a reaction profile for an exothermic catalysed and uncatalysed reaction. Label the activation energy for both rescrions.
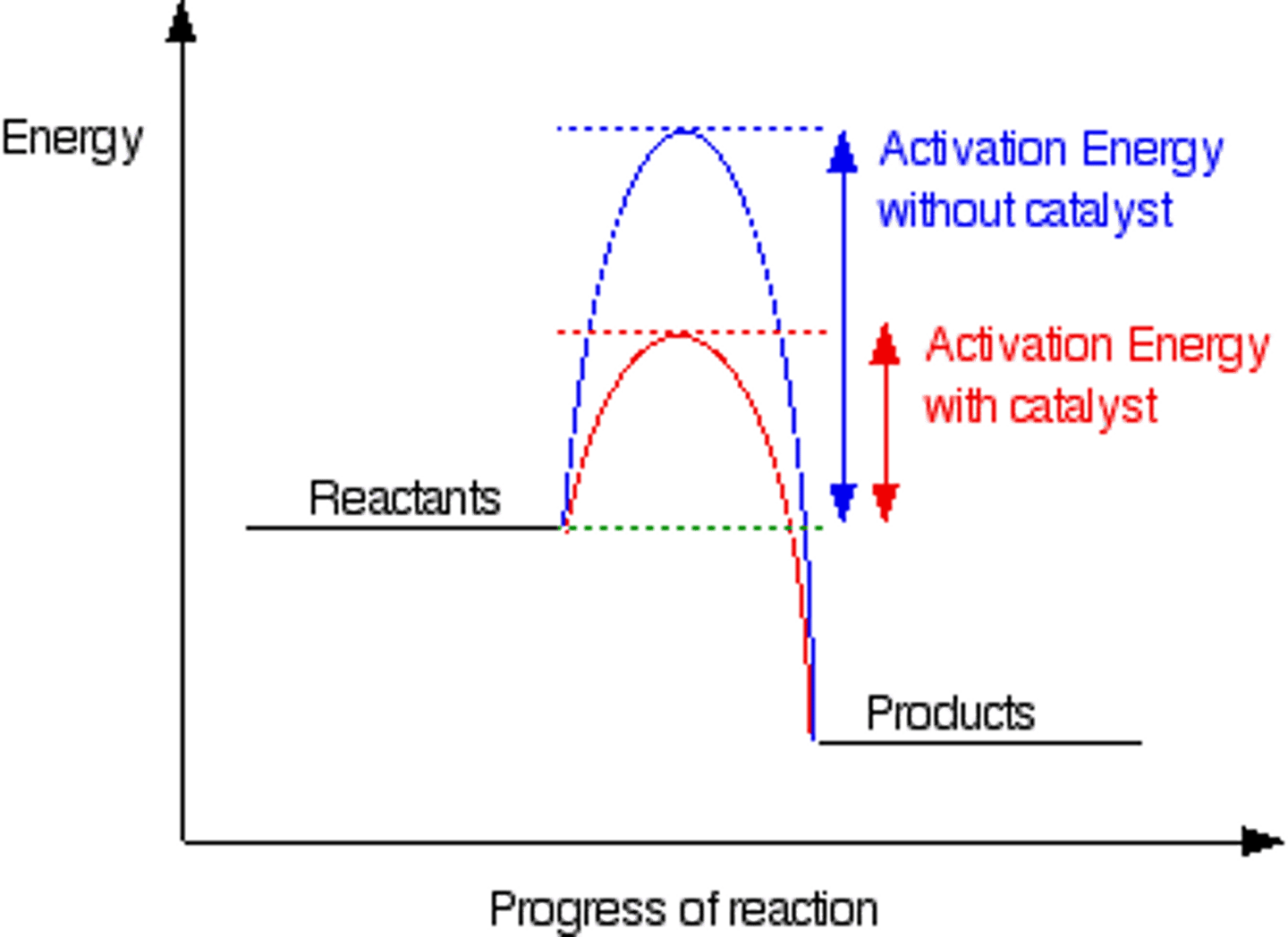
Describe how you would identify whether a substance is a catalyst
Firstly, the substance must increase rate of reaction. Secondly, the substance must not be used up or produced in the reaction and must not be in the chemical equation for the reaction.
Define reversible reactions
Reactions where products of the reaction can react to form the original reactants.
Generally, how would you change the direction of a reversible reaction.
Changing the conditions.
Write a word equation for the decomposition of ammonium chloride. Describe how you would change the direction of this reaction.
Ammonium chloride '—, ammonia + hydrogen chloride
To make the reaction go forward, heat the mixture. To make the reaction go backward, cool the mixture.
Describe reversible reactions in terms of exothermic and endothermic reactions and energy changes.
If a reversible reaction is exothermic in one direction, it is endothermic in the opposite direction. The same amount of energy is transferred in each case.
Write a word equation for the test for water. Give the colours of each solid, and state whether the reaction is exothermic or endothermic.
Hydrated copper sulphate (blue) '—, anhydrous copper sulphate (white) + water
The forward reaction is endothermic while the backward reaction is exothermic
Describe equilibrium and the conditions needed for it to occur.
When a reversible reaction occurs in apparatus which prevents the escape of reactant and products, it is reached when the forward and recerse reactions occur at exactly the same rate.
State Le Chatelier's principle
If a system is at equilibrium and a change is made to any of the conditions, then the system responds to counteract the change
Describe the effect of increasing or decreasing the concentration of a reactant or product on equilibrium.
If the concentration of a reactant is increased or if the concentration of a product is decreased , more products will be formed from reacting reactants until an equilibrium is reached.
If the concentration of a product is increased or if the concentration of a reactant is decreases, more reactants will be formed from reatring products until equilibrium is reached.
Describe the effect of increasing or decreasing the temperature on equilibrium.
If the temperature of a reaction is increased, the equilibrium will shift so that the products of the endothermic reaction will increase in concentration. If the temperature of a reaction is decreased, the equilibrium will shift so that the products of the expthermic reaction will increase in concentration.
Describe the effect of increasing or decreasing the pressure at equilibrium
If the pressure increases, the equilibrium will shift towards the side with the smaller number of gas molecules. If the pressure decreases, the equilibrium will shift to the side with the larger number of gas molecules.