Instrumental Methods Quiz 2
1/96
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
97 Terms
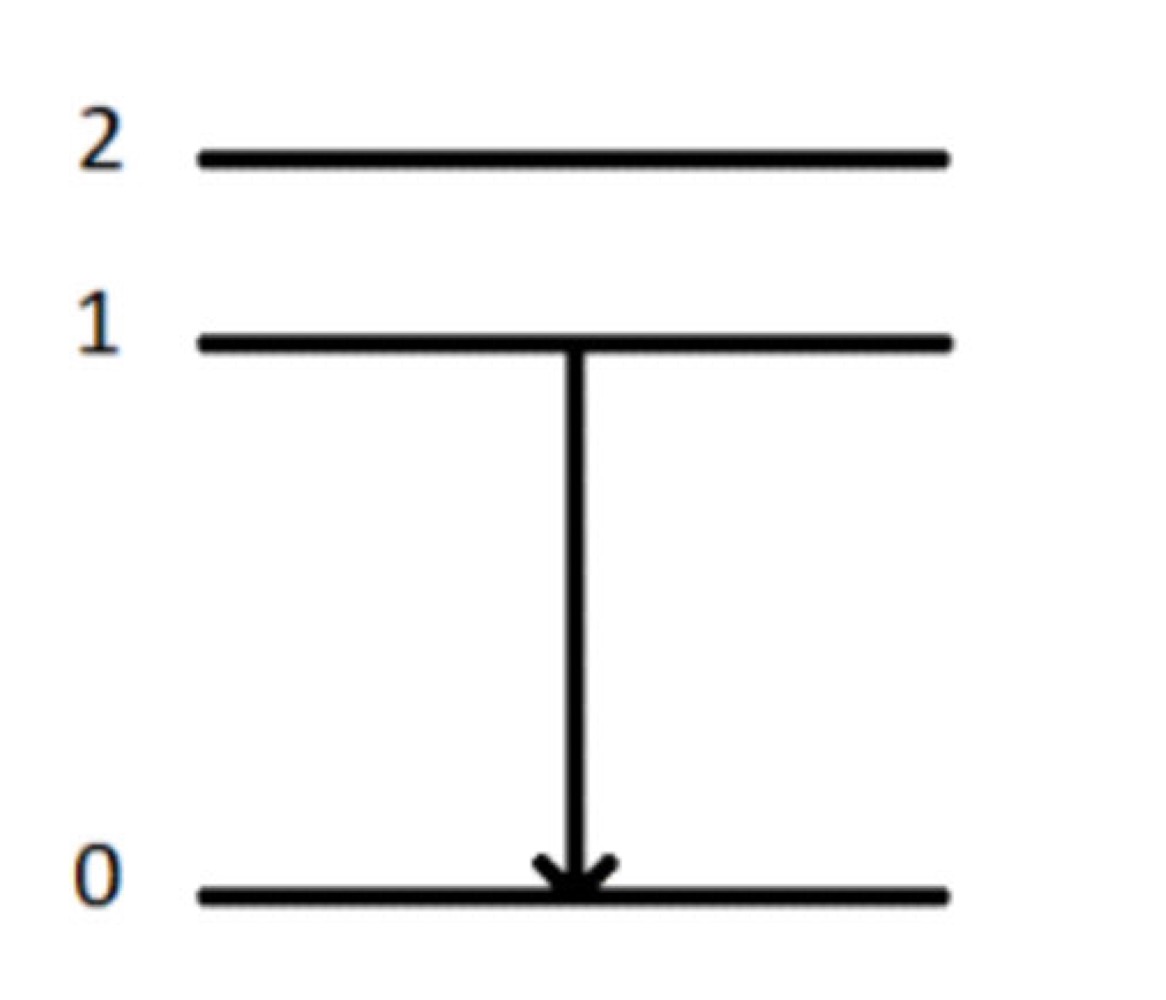
What method of spectroscopy is this?
Emission Spectroscopy
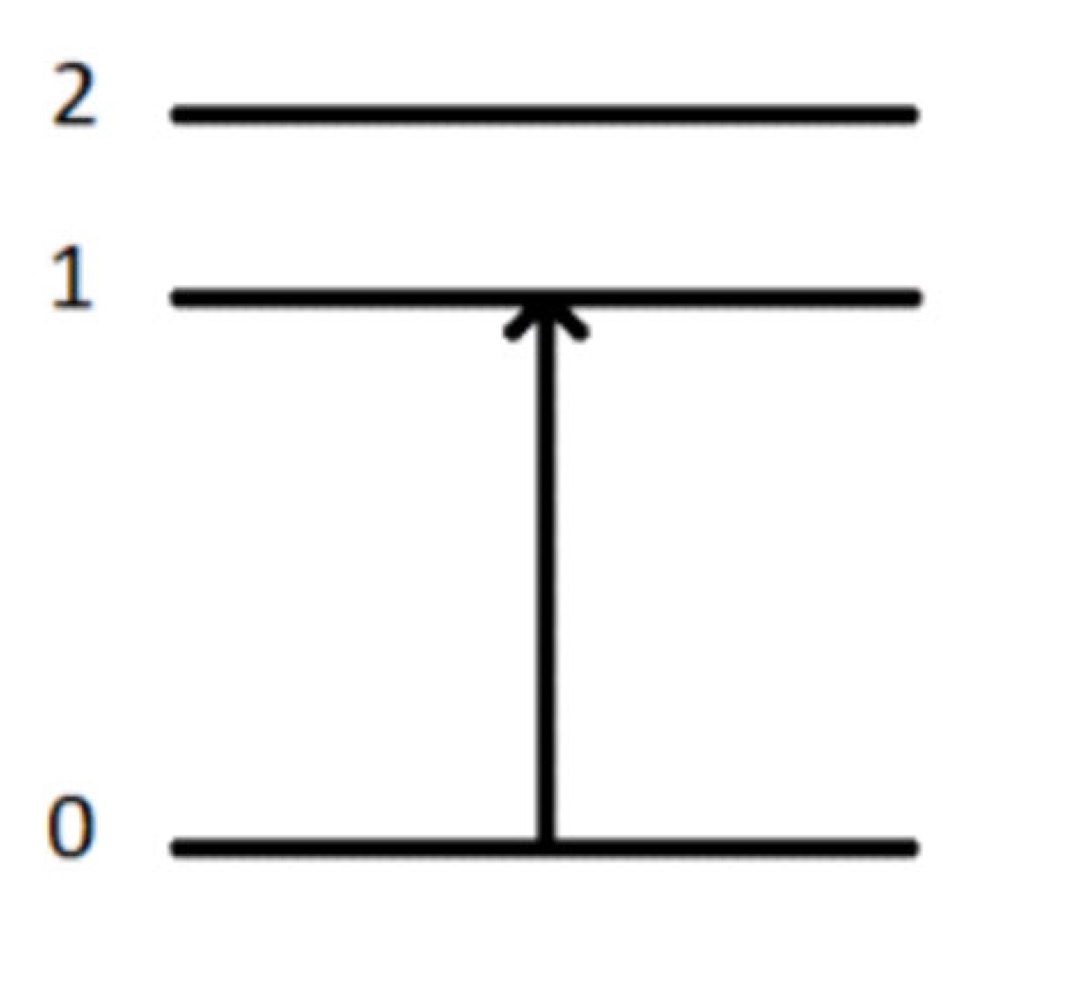
What method of spectroscopy is this?
Absorption Spectroscopy
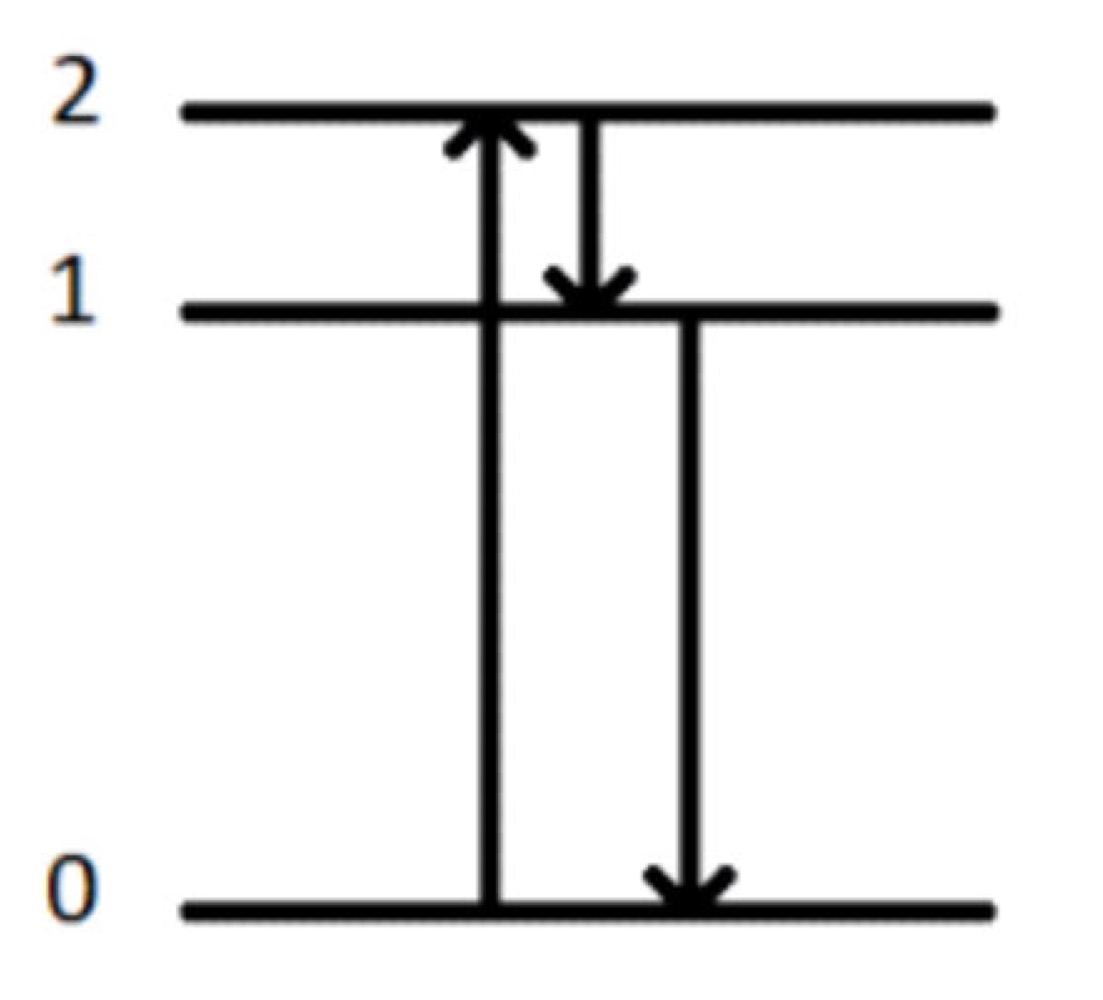
What method of spectroscopy is this?
Photoluminescene Spectroscopy
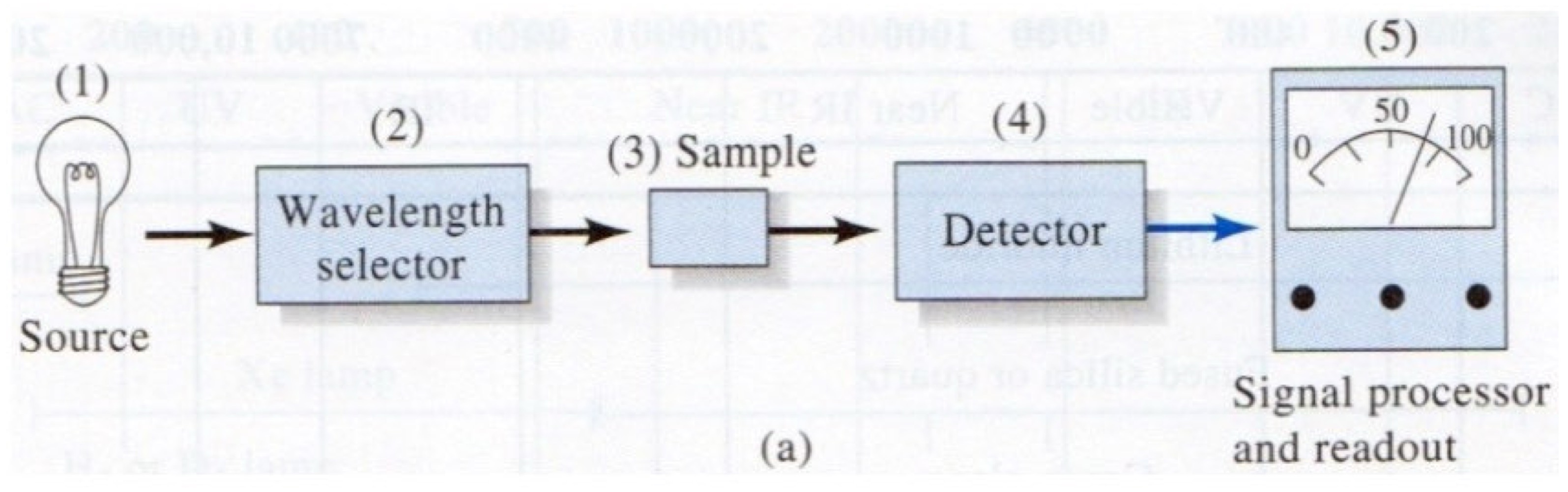
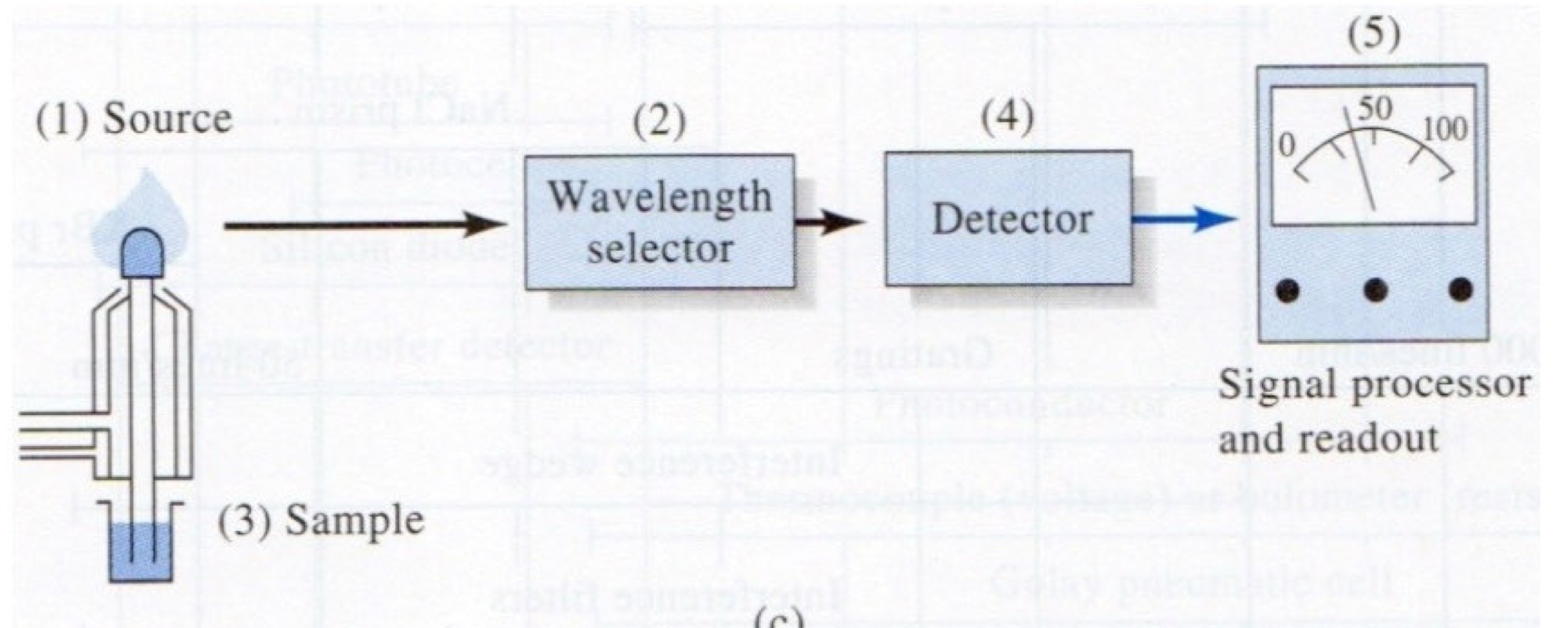
What method of spectroscopy corresponds to this image?
Absorption Spectroscopy
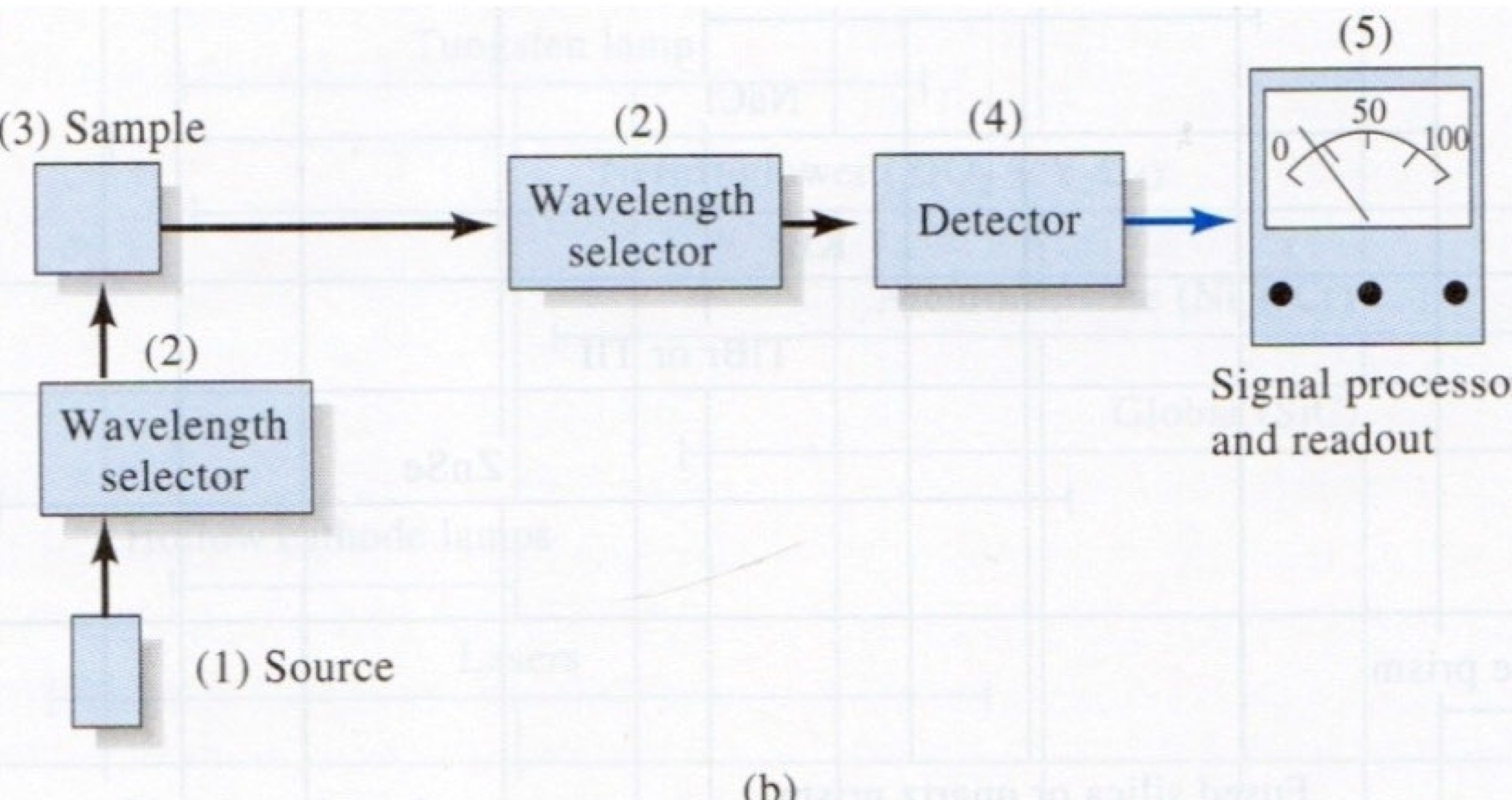
What method of spectroscopy corresponds to this image?
Emission Spectroscopy
What method of spectroscopy corresponds to this image?
Photoluminesecene Spectroscopy
An atomic absorption spectrometer uses a tungsten hollow-cathode lamp to measure the concentration of tungsten in aqueous samples. To do this, the tungsten in the lamp is in the __________ and the hollow-cathode lamp is a __________.
Gas phase, line source
A scanning spectrometer uses a tungsten filament lamp as a __________ . To do this, the tungsten in the lamp is in the __________.
Continuum source, solid phase
Stokes Raman scattering is __________ and occurs when the molecule relaxes to a higher ground vibrational state, so the emitted a photon has __________ energy and __________ frequency than the absorbed photon.
Inelastic; less; a lower
Anti- Stokes Raman scattering is __________ and occurs when the molecule relaxes to a lower ground vibrational state, so the emitted a photon has __________ energy and __________ frequency than the absorbed photon.
inelastic; more; a higher
Rayleigh scattering is __________ and occurs when the molecule relaxes to the same ground state, so the emitted a photon has __________ energy and __________ frequency than the absorbed photon.
elastic; the same; the same
Continuum or blackbody radiation depends only on the __________ of the emitting surface, not on the __________ that makes up this surface. It is produced by the large number of atomic and molecular oscillations in a thermally excited solid. Higher temperatures produce shorter wavelengths; however, these spectra drop off rapidly in the
__________region.
|
Line spectra are produced when individual atoms in the __________ phase are excited. These atoms act independently of each other; and as a result, line spectra are sharper than continuum spectra.
gas
What lasing medium does fluorescent Solutes use?
dye lasers
What lasing medium does Gaseous CO2 or N2 use?
Molecule Lasers
What lasing medium does Gaseous Ar+ and Kr+ use?
Ion Lasers
What lasing medium does gaseous He and Ne use?
Neutral Atom Lasers
What lasing medium does Crystalline Y3Al5012 doped with Nd(III) use?
Nd-YAG Lasers
What lasing medium does p-n junction metalloids use?
semiconductor diode lasers
What lasing medium does Crystalline Y3AlO3 doped with Cr(III) use?
ruby lasers
What lasing medium does Gaseous ArF, KrF, or XeF use?
excimer lasers
The term __________ is used to accurately describe the output from a wavelength selector.
effective bandwidth
An interference filter, a Fabry-Pérot filter, and an interference wedge all filter by transmitting certain bands of electromagnetic radiation and reflecting other bands of electromagnetic radiation.
true
Machined or ruled gratings are more accurate than holographic gratings made by interference patterns on a photosensitive film.
false
Holographic gratings are used to filter reflected electromagnetic radiation or transmitted electromagnetic radiation.
true
A filter, prism, or grating are possible components of a __________ .
wavelength selector
Monochromators for ultraviolet, visible, and infrared sources all use slits, lenses, mirrors, and prisms or gratings to produce electromagnetic radiation at nearly a single wavelength or to scan a continuous spectrum of wavelengths.
True
A relatively wide slit width generally lets a __________ number of wavelengths reach the detector. This __________ the likelihood of analytical interference from the sample matrix.
larger; increases
An entrance __________ is used to direct polychromatic or nearly monochromatic electromagnetic radiation from the source to the prism or grating. An exit __________ is used to direct nearly monochromatic electromagnetic radiation from the prism or grating to the sample container.
slit; slit
Radiation transducers convert electromagnetic radiation into an electrical signal and are used as detectors in optical instruments.
true
Ideally, radiation transducers have a __________ sensitivity and signal to noise ratio. It's best if they have a fast response time, and a __________ response over a __________ range of concentrations and wavelengths.
high; linear; wide
A vacuum phototube emits electrons from a photoemissive __________ . The number of absorbed __________ is proportional to the number of emitted __________.
cathode; photons; electrons
The most common optical detector is the __________ . It has a photoemissive cathode, an anode, and several other electrodes called dynodes in an evacuated quartz or glass tube.
photomultiplier tube
The photoemissive cathode in a PMT is a metal coated with Na/K/Cs/Sb, Ga/As, or other mixtures of elements that absorb photons and emit electrons. These emitted electrons are accelerated toward the __________ by a voltage that is more positive than the __________ . As a result, each electron that strikes a dynode causes the emission of several more electrons. This cascade of electrons is collected at the __________ . Ultimately, 1 photon typically produces 106 or 107 electrons.
dynode; cathode; anode
A p-type semiconductor has a __________ charge from the accumulation of __________ .
positive; holes
Under a forward bias, a positive potential is applied to the __________ semiconductor and a negative potential is applied to the __________ semiconductor. The holes and electrons flow toward the junction, recombine, and are annihilated. Therefore, current __________ in this direction.
p-type; n-type; easily flows
Under a reverse bias, a positive potential is applied to the __________ semiconductor and a negative potential is applied to the __________ semiconductor. The flow of charge carriers is away from the junction. As a result, the charge carriers are depleted near the junction and current __________ flow(s) in this direction. However, if photons with energy that is greater than the band gap are absorbed at this depleted layer, electron-hole pairs are formed, and a current is produced. This current is proportional to the intensity of the light falling on the diode.
n-type; p-type; does not easily
A photodiode array (PDA) is a transducer that allows the __________ measurement of light intensity at many wavelengths. These are commonly used in scanning __________ spectroscopy.
simultaneous; UV/Vis
A photodiode array (PDA) is a __________ dimensional array of __________ n-type and p-type semiconductors.
1; reverse-biased
Photodiode arrays (PDAs) __________ as sensitive as photomultiplier tubes, and have __________ dynamic ranges, and __________ signal to noise ratios compared to photomultiplier tubes.
are not; smaller; smaller
Photodiode array (PDAs) typically use a __________ and a __________ array of transducers.
diffraction grating; 1-D
A UV/Vis spectrometer often has 2 sources. A __________ is often used as an ultraviolet region source, and a __________ is often used as the visible region source.
deuterium or hydrogen arc lamp; tungsten filament lamp
A UV/Vis spectrometer is often a double-beam instrument with a sample cell and a reference cell. The electromagnetic radiation that leaves these cells is usually separated by wavelength with a grating or prism, and directed to a __________ detector so that the entire spectrum is measured simultaneously.
photodiode array
Charge-transfer devises (CTDs) __________ as sensitive as photomultiplier tubes, and have comparable dynamic ranges, and __________ signal to noise ratios as photomultiplier tubes.
are approximately; comparable
Charge-transfer devises (CTDs) typically use a __________ , and a __________ array of transducers.
echelle monochromator; 2-D
A monochromator with one dispersing element, such as a diffraction grating, can use a __________ array of transducers. A monochromator with two dispersing elements, such as a diffraction grating and a prism, can use a __________ array of transducers.
1-D; 2-D
IR is generally not energetic enough to ionize electrons. As a result, IR detectors generally __________ use photoionized electrons to produce a signal.
cannot
Magnesium metal (Mg) in the singlet ground state has two 3s valence electrons; that is, 1 valence electron is spin up and 1 valence electron is spin __________ . This electron spin and orbital motion create __________ magnetic field.
down; an attractive
If magnesium metal (Mg) is in the singlet excited state with one 3s valence electron and one 3p valence electron, then these valence electrons have __________ and the 3p orbitals __________ .
opposite spins; are not split and have the same potential energy
If magnesium metal (Mg) is in the triplet excited state with one 3s valence electron and one 3p valence electron, then these valence electrons have __________and the 3p orbitals __________ .
the same spin; are split and have 3 slightly different energies
Atomic spectrometry is based on the electronic transitions from the absorption or emission of radiant energy by individual gaseous atoms. Vibrational and rotational energy is not involved. As a result, atomic spectra consist of very narrow lines, in contrast to the wide band of molecules in solution.
true
Name the broadening factor: If a gaseous atom rapidly moves toward a detector, the frequency of an absorbed or emitted photon increases. If a gaseous atom rapidly moves away from a detector, the frequency of an absorbed or emitted photon decreases.
doppler broadening
Name the broadening factor: The fundamental limit in the accuracy of calculating the energies of a ground and excited electronic state, and the time that an electron resides in these ground and excited electronic states.
the Heisenberg uncertainty principle
Name the broadening factor: the splitting of atomic absorption and emission lines by an external magnetic field
Zeeman splitting
Name the broadening factor: The splitting of atomic absorption and emission lines by an external electric field.
Stark broadening
Name the broadening factor: The collisions of absorbing or emitting atoms with other atoms at high temperature produces small changes in the potential energy levels of the electronic transitions that give a range of absorbed or emitted wavelengths.
pressure broadening
The temperatures used by most atomic spectroscopic methods range from 1,200° C to 6,000° C. At these temperatures, a __________ of atoms are in their lowest possible energy state, the ground state. And __________ atoms are in an excited state
large majority; very few
Electrothermal Vaporization
An electric current is used to vaporize a sample of solid, liquid, or solution. This vapor is carried to an atomizer by an inert gas.
Laser ablation
A Nd-YAG (neodymium ion in a host crystal of yttrium aluminum garnet) or excimer laser is used to aerosolize a small amount of particulate matter from a solid metal sample. This particulate matter is carried to an atomizer by an inert gas.
spark or arc ablation
An electrical discharge is applied to an electrically conducting solid. A small amount of particulate matter is aerosolized and carried to an atomizer by an inert gas.
Pneumatic nebulization
A high-pressure stream of gas is used to aspirate a sample of solution or suspension through a capillary tube and into an atomizer.
ultrasonic nebulizer
A mechanical stress is applied to piezoelectric crystal; as a result, this crystal vibrates at a high frequency and converts a liquid sample to an aerosol. This aerosol is carried to an atomizer.
What sample introduction is used for solid powders?
direct insertion
What sample introduction is used for solutions or suspensions?
pneumatic nebulization
What sample introduction is used for solid metals
laser ablation
What sample introduction is used for electrically conducting solids?
glow-discharge sputtering/and arc or spark ablation
What sample introduction is used for solids, liquids, or solutions?
electrothermal vaporization
What sample introduction is used for solutions?
hydride generation/and ultrasonic nebulization
The continuous introduction of sample into a flame or plasma produces individual atoms in the gas phase. The nebulizer converts the sample solution into a __________ . The high temperature of the flame or plasma causes the solvent to evaporate and produces a __________ . Further heating by the flame or plasma produces __________ .
spray; dry aerosol; free atoms
The 2 most common methods of sample atomization in atomic absorption spectrometry (AAS), atomic fluorescence spectrometry (AFS), and atomic emission spectrometry (AES) are __________ atomization and __________ atomization.
electrothermal vaporization and flame
A flame atomizer __________ the sample into a fine spray of droplets. The solvent is __________ in the flame by desolvation.
nebulizes; evaporated
During introduction of sample by flame atomization, the sample is converted into an aerosol, a __________ of fine solid particles or liquid droplets that are __________ in a gas. The aerosol is __________ into gaseous molecules and gaseous atoms.
colloid; suspended; volatillized
In flame atomization, in the volatilized sample, some of these gaseous atoms are __________ into gaseous cations and gaseous electrons. A small fraction of these gaseous molecules, atoms, and cations are __________ into higher energy states. These higher energy states __________ to give molecular, atomic, and ionic emission spectra.
ionized; electronically excited; decay
What gases are considered a fuel?
hydrogen, acetylene, natural gas
What gases are considered an oxidant?
oxygen, nitrous oxide, air
Since emission and absorption spectroscopy measure __________, hot flames decrease the detection limit of these easily ionized elements. High temperature flames prevent interference from the production of __________ in the flame.
un-ionized atoms; heat-stable oxides
What region of a flame: Has an excess of fuel. The oxidant is the limiting reagent for combustion in this region. This region is rarely used in flame spectroscopy.
primary combustion zone
What region of a flame: The combustion reaction is approximately stoichiometric in this region. The hottest part of a flame. Free atoms are dominant. This region is widely used in flame spectroscopy.
interzonal region
What region of a flame: has an excess of oxidant. The fuel is the limiting reagent for combustion in this region. This region is rarely used in flame spectroscopy.
secondary combustion zone
__________ atomization is generally the more sensitive of these 2 sample introduction methods.
Electrothermal
Flame methods convert __________ of the analyte to atomic vapor. In contrast, electrothermal atomizers convert __________ of the analyte to atomic vapor. Therefore, flame methods have detection limits that are 100 to 1000 times __________ than electrothermal atomizers.
as little as 0.1%; nearly 100% higher
A micropipette is used to introduce the sample to the inside of a cylindrical graphite tube. This sample is introduced through a hole in the side of this tube and placed on an L’vov platform. This tube is approximately 5 cm long by 1 cm in diameter. Each end of the tube is held in place with an electrical contact. These contacts are held in place by a water-cooled metal jacket. An __________ stream of inert gas excludes air and carries away vapors during the desolvation and ashing steps. An __________ stream of inert gas prevents air from incinerating the heated graphite tube.
internal; external
The sample is first __________ and then __________ while on the L’vov platform by a gradually increasing temperature. Finally, the sample is __________ by a rapidly increasing temperature.
dried; ashed; atomized
Cold vapor atomization is used only for the determination of mercury. Nitric (HNO3) and sulfuric (H2SO4) acids are used to oxidize the analyte to Hg2+. The HNO3 is an __________ agent. That is, the HNO3 gets __________ .
oxidizing; reduced
Then stannous chloride dihydrate (SnCl2·2H2O) is used to reduce the Hg2+ to Hg. The SnCl2·2H2O is an __________ agent. That is, the SnCl2·2H2O gets __________ .
An inert gas is used to carry this Hg into a tube where the absorbance is measured at 253.7 nm. The longer the tube, the __________ the detection limit.
reducing; oxidized; lower
The hollow-cathode lamp is the most common source for atomic absorption and atomic fluorescence spectrometry. The anode is made out of tungsten. The cathode is cylindrical and is made out of the same metal as the analyte. These electrodes are sealed in a glass tube with neon (Ne) or argon (Ar) at 1 to 5 torr. The noble gas is ionized at approximately 300 volts. The gaseous Ne or Ar cations migrate to the __________ charged cathode, and the gaseous electrons migrate to the __________ charged anode. Some of these gaseous cations dislodge and vaporize metal atoms from the cathode. The gaseous analyte atoms are in equilibrium with ionized analyte cations; the gaseous electrons shift this equilibrium toward gaseous analyte __________ . Some of these analyte atoms in this vapor are in excited electronic states and emit discrete lines of electromagnetic radiation when they decay to their ground electronic state. Finally, the analyte atoms are re-deposited on the cylindrical cathode.
negatively; positively; atoms
A hollow-cathode lamp uses __________ to ionize a noble gas in its sealed glass tube. An electrodeless discharge lamp uses __________ to ionize a noble gas in its sealed glass tube.
an electric current; electromagnetic radiation
In a __________ instrument, a mirrored chopper directs a sample beam through the flame and a reference beam around the flame. Then each beam typically goes to a monochromator and a photomultiplier tube. The __________ beam signal is subtracted from the __________ beam signal.
double-beam; reference; sample
Both vanadium (V) and aluminum (Al) have an atomic line at 308.2 nanometers; this is an example of __________ .
spectral interference
Calcium ion (Ca2+) from a sample of drinking water stays in the +2 oxidation state during flame atomic spectrometry; this is an example of __________ .
ionization interference
Calcium (Ca) reacts with sulfate (SO42−)in the sample matrix to produce calcium sulfate (CaSO4) during flame atomic spectrometry; this is an example of __________ .
interference from a refractory compound
Blank correction, continuum-source background correction, and Zeeman-effect background correction are used to correct __________ .
spectral interference
A continuum-source background correction for broadband absorption in the __________ region is made with a hydrogen (H2) or deuterium (D2) continuum source. This background correction in the __________ region is made with a tungsten (W) continuum source. A chopper is used to alternatively direct radiation from an analyte hollow-cathode lamp __________ source and the continuum source through the flame or electrothermal atomizer, the monochromator, and the detector.
ultraviolet; visible; line
The continuum source is an accurate estimate of absorption from the __________ over a relatively __________ spectral bandwidth.
sample matrix; broad
The line source is an accurate estimate of absorption from the __________ over a relatively __________ spectral bandwidth.
analyte and sample matrix; narrow
The processed signal from the __________ source is subtracted from the processed signal from the __________ source to give the analyte concentration.
continuum; line
An alternating magnetic field is used for Zeeman-effect background correction. This alternating field is focused on the atomic vapor in an electrothermal atomizer. In the simplest case, this alternating field splits a single atomic absorption line into 3 lines: a π line at the same energy as the original absorption line, a higher energy σ line, and a lower energy σ line. The total absorption is measured with the magnetic field off. The background absorption is measured with the magnetic field on and the π line removed with a polarizer; therefore, only the background absorption is measured. Therefore, signal when the magnetic field is __________ minus the signal when the magnetic field is __________ is used to measure the analyte concentration.
off; on