particle nature
1/13
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
14 Terms
describe a solid
particles have low energy and cannot overcome the strong forces of attraction holding them together
describe the movement a solid
Can only vibrate around a fixed point
vibrational and rotational motion
describe a liquid
particles have large amount of energy and can overcome the strong forces of attraction to some extent
describe the movement of a liquid
they can move freely around each other whilst in close proximity
have vibrational, rotational and translational motion
describe a gas
particles have large amounts of energy and can almost completely overcome the strong forces of attraction
describe the movement of gas
they move rapidly and randomly into available space
have vibrational, rotational and translational motion
define the kelvin scale
if temperature decreases, the average kinetic energy of particles decreases
absolute zero (0K) is lowest possible temperature as all motion of particles has stopped
Converting Celsius into kelvin
add 273
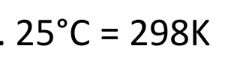
Physical Changes: State of Matter
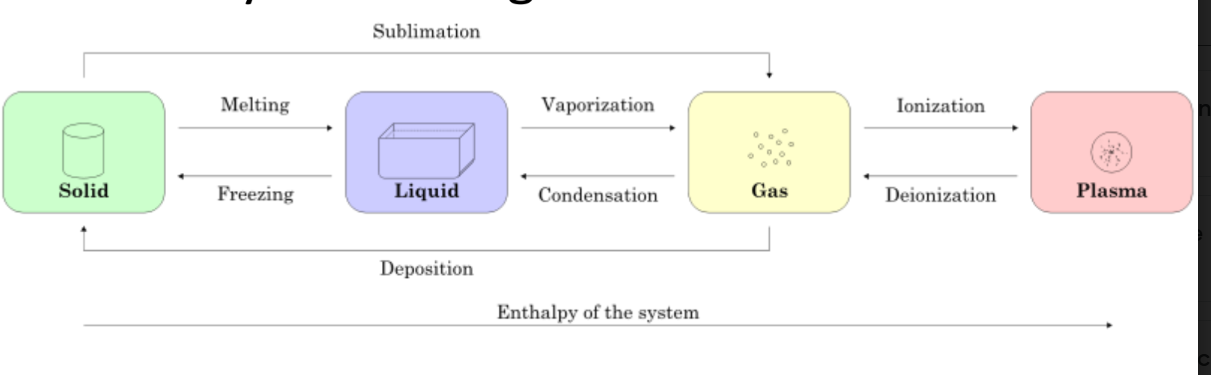
Which physical changes are exothermic?
condensation and freezing (deionization)
Which physical changes are endothermic?
melting and vaporization (ionization )
evaporation definition
Can occur at any temperature and only at the surface of a liquid
boiling definition
occurs at fixed temperatures when the vapour pressures equals the external pressure
At different external pressures , the boiling point of a liquid will be different
Characteristics of matter
Made up of particles such as atoms, molecules or ions
Occupies a volume in space
Has a mass
Particles are in constant motion