Chemical bonds
1/99
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
100 Terms
Outer most shell in groups 1,2, 7 and 8
In group 1, the outer most shell is s1, goes up in 1
In group 2, the outer most shell is s2, goes up in 1
In group 7, the outer shell is s2 p5, goes up in 1
In group 8, the outer shell is s2 p6, goes up in 1
Valence shell
Atoms whose outer (valence) electron energy levels hold a complete set of electrons are substantially more stable than those which do not.
In each group the elements have the same outer energy level electron configuration, and therefore the same valency.
Group 1 elements require the input of a relatively small amount of energy to lose an electron, i.e. to ionise.
It is extremely difficult to remove an electron from a Group 7 element; these are close to a stable octet and complete outer electron energy level and therefore have a strong tendency to acquire electrons.
Electrons are added one at a time, moving from left to right across a period. As this happens, across a row the electrons in the outermost energy level experience increasingly strong nuclear attraction and become closer and more tightly bound.
Furthermore, moving down a group, the outermost electrons become less tightly bound to the nucleus.
This happens because the number of filled principal energy levels increases downward in each group, and so the outermost electrons are more shielded from the attraction of the nucleus.
These trends explain the periodicity observed with respect to atomic radius, ionisation energy, electron affinity and electronegativity in moving from left to right across a period.
Octet rule
Electrons obey the octet rule (tendency of electrons to prefer to have 8 electrons in the outer shell).
Elements tend to gain or lose valence electrons to achieve a stable octet formation.
Stable octets are seen in the inert gases, Group 8 of the periodic table.
Ionic compounds
Electrons of atoms of a metal are transferred to atoms of nonmetals, which form positive and negative ions, creating strong attractive forces
Strongest of the attractive forces found in a compound
Most ionic compounds are solids at room temperature
Large amounts of energy are needed to overcome the strong attractive forces between positive and negative ions
Molecular compounds
Atoms of two or more nonmetals share one or more valence electrons. The shared atoms are held together by covalent bonds that form a molecular.
Number of valences in group 1
1 electron in valence shell
Lose 1 or gain 7 to achieve noble gas configuration
Losing 1 energetically favourable so 1 valence
Number of valences in group 2
2 electrons in valence shell
Lose 2 or gain 6 to achieve noble gas configuration
Losing 2 energetically favourable so 2 valences
Number of valences in group 3
3 electrons in valence shell
Lose 3 or gain 5 to achieve noble gas configuration
Losing 3 energetically favourable so 3 valences
In higher periods: full s shell stabilise too, also 1 valence
Number of valences in group 4
4 electrons in valence shell
Lose 4 or gain 4 to achieve noble gas configuration
Losing 4 or gaining can be energetically favourable so 4 valences either way
In higher periods: full s shell stabilise too: also 2 valences
Number of valences in group 5
5 electrons in valence shell
Lose 5 or gain 3 to achieve noble gas configuration
Both processes occur: minimum 3 valences and maximum 5 valences
Number of valences in group 6
6 electrons in valence shell
Lose 6 or gain 2 to achieve noble gas configuration
Loss or gain possible: minimum 2 valences and maximum 6 valences
Number of valences in group 7
7 electrons in valence shell
Lose 7 or gain 1 to achieve noble gas configuration
Loss or gain possible: minimum 1 valences and maximum 7 valences
Number of valences in group 8
2 electron in valence shell = 2 valences
1B = 1 electron fills d shell = 1 valence
Maximum and minimum valences
Max: group number
Min: 8 - group number
Positive ions
Ions with positive charges are formed by the loss of electrons.
Ionisation energies of metals of groups 1, 2 and 3 are low so metal atoms readily lose their valence electrons.
Negative ions
Ions with negative charges are formed by the gain of electrons.
Ionisation energies of groups 5, 6 and 7 are high so nonmetal atoms gain one or more valence electron to obtain a stable electron arrangement.
Valences of H, F and O
H and F always 1
O always 2
Valences of main group metals
Usually group number
Higher homologues in Groups 3, 4 and 5 and also 2 less
Valences of transition metals
All 2 valencies: Ti: 2, Cr: 2, Fe: 2, Zn: 2
Groups 3 to 7 also maximal 3 to 7, intermediate numbers in steps of 1 e.g. Mn: 2, 3, 4, 7
Group 8 also 3: Fe: 3, Co: 3, Ni: 3
Group 1 also 1: Cu: 1, Ag:1, Au: 1
Valences of non-metals, groups 5, 6 and 7
Usually (8 - group number) in combination with other metals e.g. N: 3, O: 2, F, Cl: 1
Maximum number of valences in combination with other non-metals except H (co-ordinate covalent bond) e.g. HNO3: 5, H2SO4: 6, HClO4: 7
Intermediate vacancies if, possible, in steps of 2: HCl/HOCl:1, HClO2:3, HClO3: 5, HClO4: 7
Arrangement of NaCl
The larger Cl- ions are arranged in a 3D structure in which the smaller Na+ ions occupy the space between the Cl- ions
Every Na+ ion is surrounded by six Cl- ions, and every Cl- ion is surrounded by six Na+ ions.
Thus, there are many strong attractions between the positive and negative ions, which account for high melting points of ionic compounds.
Electronegativity
Ability to attract the shared electrons in a chemical bond.
Nonmetals have higher electronegativity because they have a greater attraction for electrons than metals.
Nonpolar covalent bond
A bond between atoms with identical or very similar electronegativity values
Electronegativity difference of 0 to 0.4
Polar covalent bond
Covalent bond between atoms with different electronegativity values where electrons are shared unequally
The electron cloud is unsymmetrical
The shared electrons are attracted to the more electronegative atom, which makes it partially negative because of the negatively charged electrons around that atom
The atom with the lower electronegativity becomes partially positive because of the lack of electrons to that atom
A bond becomes more polar as the electronegativity difference increases
Dipole
Polar covalent bond that has a separation of charges
The positive and negative ends of the dipole are indicated by the lowercase Greek letter delta with a positive or negative sign
Electronegativity difference and types of bonds
Non polar covalent bond
0.0 to 0.4 electronegativity difference
Electrons shared equally
Polar covalent bond
0.5 to 1.8 electronegativity difference
Electrons shared unequally
Ionic bond
1.9 to 3.3 electronegativity difference
Electrons transferred
Polyatomic ions
Group of covalently bonded atoms that have an overall ionic charge
Most polyatomic ions consist of a nonmetal such as phosphorus, sulphur, carbon, or nitrogen covalently bonded to oxygen atoms
Coordinate bond
covalent bond in which the electrons come from the same atom
single covalent bond
double covalent bond
a type of covalent bond where two pairs of electrons are shared between two atoms - form when the number of valence electrons is not enough to complete the octets of all the atoms in the molecule
triple covalent bond
a type of covalent bond where three pairs of electrons are shared between two atoms, form when the number of valence electrons is not enough to complete the octets of all the atoms in the molecule
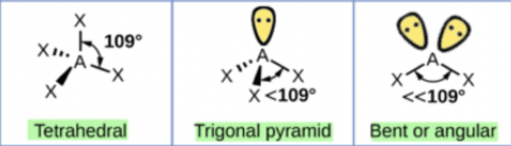
Valence Shell Electron Pair Repulsion Theory (VSEPR theory)
the electron groups (lone pair, single/double/triple bond) are arranged as far apart as possible around the central atom to minimise the repulsion between their negative charges
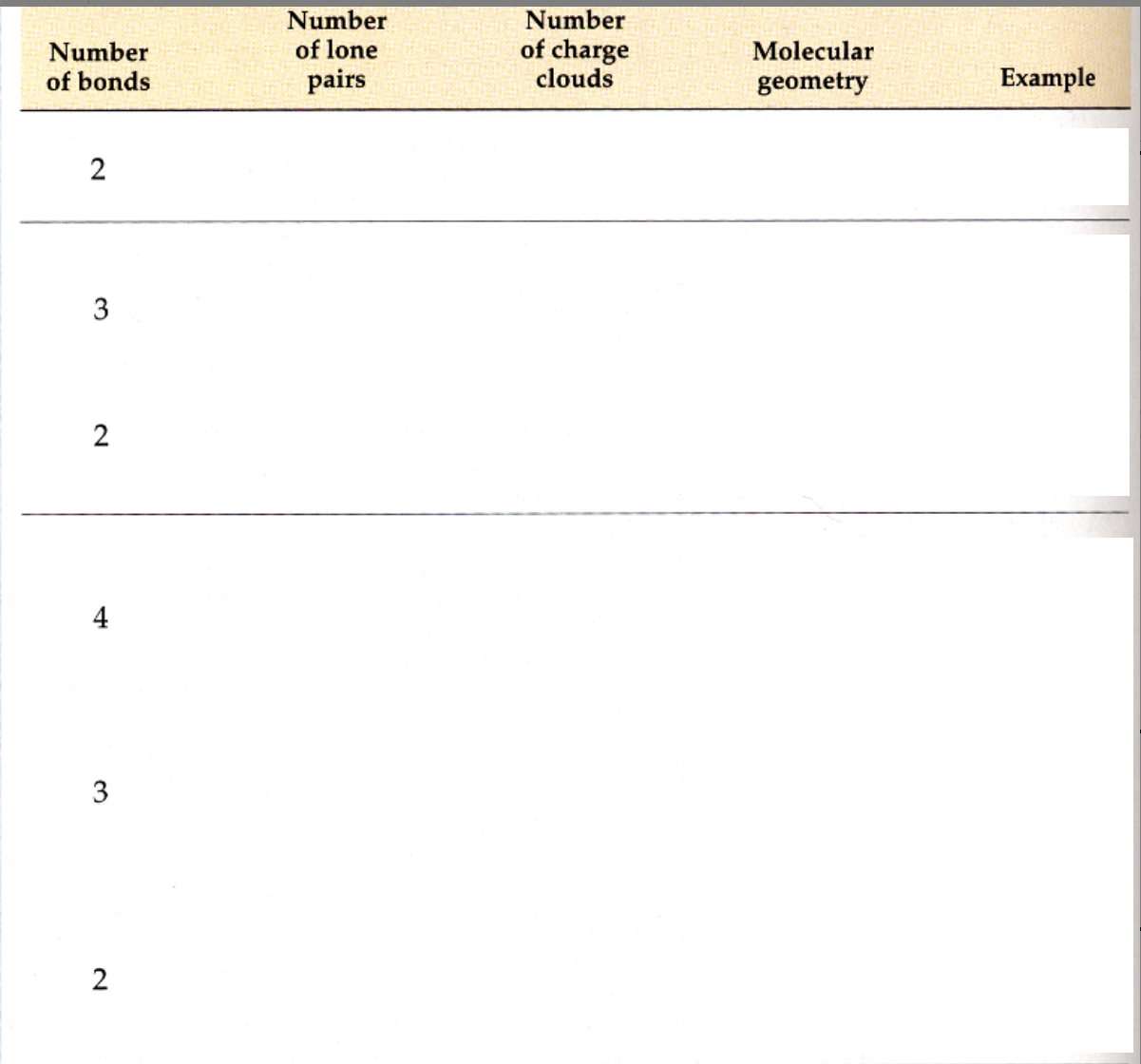
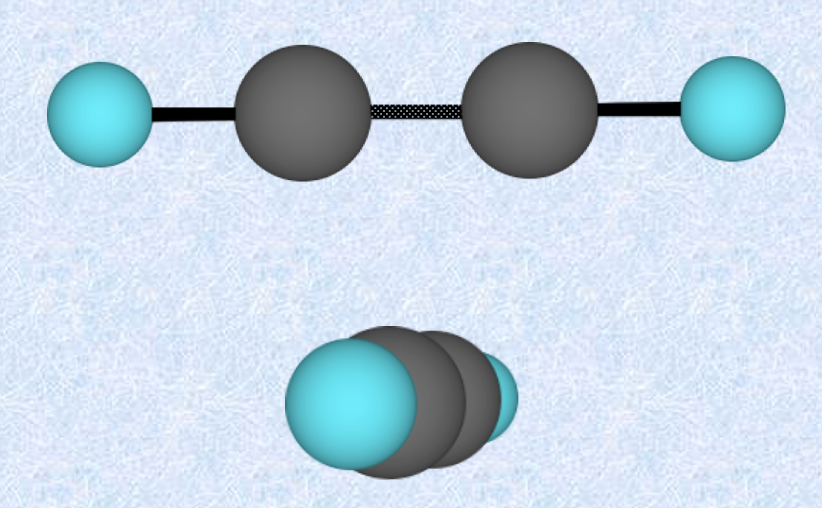
Linear shape
Minimal repulsion between two electron groups happens when the groups are on opposite sides of the central C atom
Bond angle of 180 degrees
Example: CO2
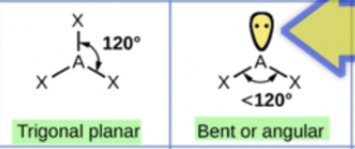
Trigonal planar/planar triangular shape
Trigonal planar/planar triangular:
Minimum repulsion between 3 electron groups occurs when they are as far apart as possible around the central C atom
Bond angles of 120 degrees
Example: BF3
Bent:
One of the electron groups can be a lone pair
Example: SO2
Shape of the SO2 molecule is determined by the O atoms bonded to the central S atom, which gives the SO2 molecule a shape that is bent and a bond angle of 120 degrees
When the central atom has more electron groups than bonded atoms, the shape and the electron-group geometry have different names
Tetrahedral shape
Tetrahedral:
Minimum repulsion between 4 electron groups occurs when they are as far apart as possible around the central C atom
109 degree bond angles
Example: CH4
Trigonal pyramidal:
One or more of the electron groups are lone pairs of electrons so the central atom is only attached to two or three atoms
Example: NH3
One of the 4 electron groups in NH3 is a lone pair so the shape is determined by the H atoms bonded to the central N atom
Bond angle of 109 degrees
Bent:
Example: H2O
4 electron groups with 2 of them being lone pairs
Shape of H2O is determined by the two H atoms bonded to the central O atom so the shape of H2O is bent
Bond angle of 109 degrees
Valence bond theory
Valence Bond Theory explains chemical bonding through the overlap of atomic orbitals and the pairing of electrons.
Orbital Overlap: Bonds form when atomic orbitals overlap; greater overlap = stronger bonds.
Bond Formation: Electrons pair with opposite spins in overlapping orbitals.
Hybridization: Atomic orbitals mix to form hybrid orbitals (sp,sp2,sp3sp,sp2,sp3) to explain molecular shapes.
Types of Bonds:
Sigma (σσ) Bonds: Head-to-head overlap.
Pi (ππ) Bonds: Side-to-side overlap.
Bond Directionality: Explains the specific geometries of molecules.
Strengths:
Describes bond strength and geometry.
Explains hybridization and molecular shapes.
Limitations:
Cannot explain magnetic properties (e.g., O2O2).
Struggles with delocalized systems and complex bonding (e.g., benzene, transition metals).
Examples: Water (H2OH2O), Methane (CH4CH4).
Molecular orbital theory
Molecular Orbitals are formed when atomic orbitals combine, spreading over the entire molecule and influencing its bonding and properties.
Key Points:
Formation:
Atomic orbitals of similar energy combine to form molecular orbitals.
Number of MOs = Number of atomic orbitals combined.
Types of MOs:
Bonding Orbitals: Lower energy, stabilize the molecule.
Antibonding Orbitals: Higher energy, destabilize the molecule (∗∗ symbol, e.g., σ∗σ∗).
Bond Order:
Bond Order=Electrons in Bonding MOs−Electrons in Antibonding MOs2Bond Order=2Electrons in Bonding MOs−Electrons in Antibonding MOs.
Determines bond strength and stability.
Electron Filling:
Follows the Aufbau Principle, Pauli Exclusion Principle, and Hund’s Rule.
Lower-energy orbitals fill first.
Examples of MO Diagrams:
H2H2: Bond Order = 1 (stable molecule).
He2He2: Bond Order = 0 (no bond formation).
Key Features:
Sigma (σσ) Orbitals: Head-to-head overlap.
Pi (ππ) Orbitals: Side-to-side overlap.
Advantages:
Explains properties like magnetism (e.g., paramagnetic O2O2).
Useful for delocalized bonding (e.g., benzene).
Limitations:
More complex than Valence Bond Theory for simple molecules.
Hybrid orbitals
Hybrid Orbitals are formed by the mixing of atomic orbitals (e.g., ss, pp, dd) on the same atom to explain molecular shapes and bond angles.
Key Points:
Why Hybridization?
Explains observed molecular geometries (e.g., tetrahedral CH4CH4).
Types of Hybridization:
spsp: Linear geometry (e.g., C2H2C2H2), bond angle = 180°.
sp2sp2: Trigonal planar geometry (e.g., C2H4C2H4), bond angle = 120°.
sp3sp3: Tetrahedral geometry (e.g., CH4CH4), bond angle = 109.5°.
sp3dsp3d: Trigonal bipyramidal geometry (e.g., PCl5PCl5).
sp3d2sp3d2: Octahedral geometry (e.g., SF6SF6).
Key Features:
Number of hybrid orbitals = Number of orbitals combined.
Shape and directionality of hybrid orbitals determine molecular geometry.
Example:
Methane (CH4CH4): sp3sp3 hybridization forms 4 equivalent tetrahedral bonds.
Advantages:
Predicts molecular shapes.
Explains equivalent bond energies (e.g., in CH4CH4).
Limitations:
Does not explain magnetic properties or delocalized bonding (e.g., O2O2, benzene).
Van der Waal forces
There is a temporary difference in distribution of binding electrons which causes temporary partial positive and negative charges, which attract the opposite charge. Weakest of intermolecular forces - attraction dependent on contact area
Dipole-dipole attractions
all polar molecules are attracted to each other by dipole-dipole attractions
since polar molecules have dipoles, the positively charged end of the dipole in one molecule is attracted to the negatively charged end of the dipole in another molecule
Structure of water
water less dense when solid
solid water floats on liquid water
water bodies freeze from top down
essential for evolution of life
Hydrogen bonds
polar molecules containing hydrogen atoms bonded to highly electronegative atoms of nitrogen, oxygen or flourine form especially strong dipole-dipole attractions
occurs between the partially positive hydrogen atom in one molecule and the partially negative nitrogen, oxygen or flourine atom in another molecule
strongest type of attractive forces between polar covalent molecules
they are attractions between polar molecules and not bonds that hold molecules together
States of matter
Gas - individual molecules feel little attraction for one another and are free to move about
Liquid - individual molecules are attracted to one another but can slide over each other
Solid - individual molecules are strongly attracted to one another and cannot move around
Dispersion forces
very weak attractions that are the only intermolecular forces that occur between nonpolar molecules
usually the electrons in a nonpolar covalent molecule are distributed symmetrically but the movement of electrons may place more of them in one part of the molecule than another, which forms a temporary dipole
these momentary dipoles align the molecules so that the positive end of one molecule is attracted to the negative end of another molecule
make it possible for nonpolar molecules to form liquids and solids
Balanced chemical equations
Ratio in which reactants combine and correpsonding ratio of products. Also corresponds to ratio of moles of reactants and products
Mass percent concentration

Volume percent concentration
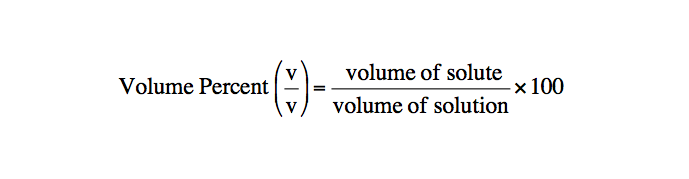
Mass/Volume Percent Concentration
Mass/volume percent (m/v) = grams of solute/milliliters of solution x 100%
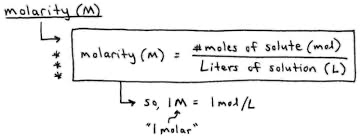
Molarity concentration
Dilution concentration equation
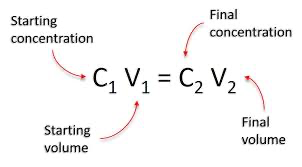
Dilution of solutions
Solvent, usually water, is added to a solution, which increases the volume
As a result, the concentration of the solution decreases
Although the addition of solvent increases the volume, the amount of solute does not change; it is the same in the concentrated solution and the diluted solution
Grams or moles of solute = grams or moles of solute
Concentrated solution Diluted solution
Heating curve
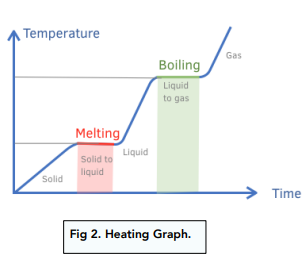
First diagonal line indicates the warmuing of a solid as heat is added.
When the melting temperature is reached, a horizontal line, or plateau, indicates that the solid is melting.
As melting takes place, the solid is changing to liquid without any change in temperature.
When all the particles are in the liquid state, adding more heat will increase the temperature of the liquid.
Once the liquid reaches its boiling point, a horizontal line indicates that the liquid changes to gas at constant temperature. Because the heat of vapourisation is greater than the heat of fusion, the horizontal line at the boiling point is longer than the horizontal line at the melting point.
When all the liquid becomes gas, adding more heat increases the temperature of the gas.
Descriptions of states of matter
Pressure, p
Pascal, 1 Pa = Newton/m2
Volume, V
Unit: cubic metre, 1m3 =1000L
Temperature, T
Unit: Kelvin, K = 273.15 degrees Celsius
Amount, n
Unit: mole, 1 mol = 6.02 × 1023
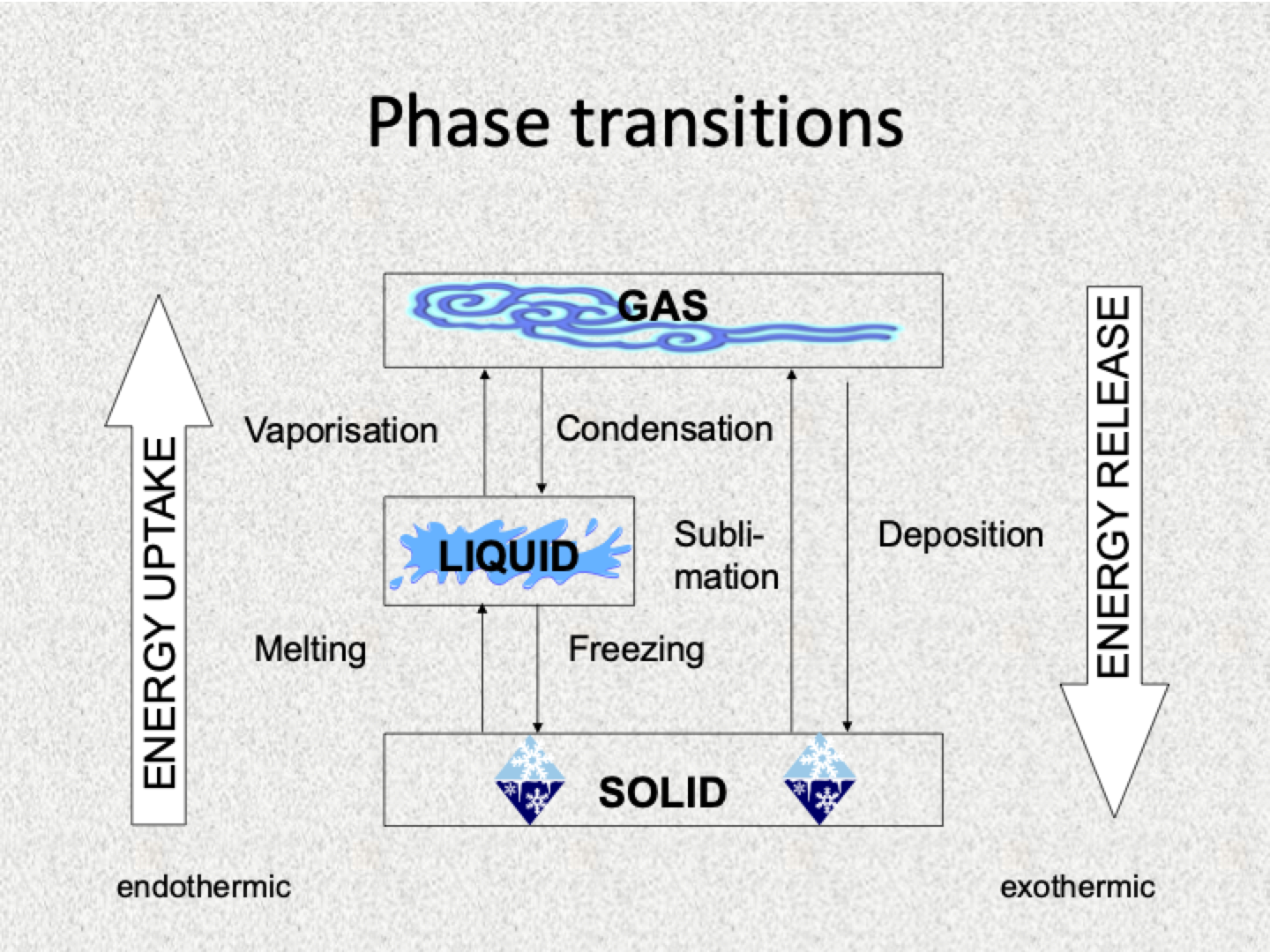
Melting
Changing from a solid to a liquid
Heat is added to a solid, particles move faster
Particles of a solid gain sufficient energy to overcome the attractive forces that hold them together
The particles in the solid separate and move about in random patterns
Freezing
Changing from liquid to solid
If the temperature of a liquid is lowered, kinetic energy is lost, the particles slow down, and attractive forces pull the particles close together.
Heat of fusion
During melting, the heat of fusion is the energy that must be added to convert exactly 1g of solid to liquid at the melting point
Sublimation
the particles on the surface of a solid change directly to a gas without going through the liquid state
Deposition
gas particles change directly to a solid
Evaporation
water particles with sufficient energy escape from the liquid surface and enter the gas phase
the loss of the hot water particles removes heat, which cools the liquid water
as heat is added, more and more water particles evaporate
Boiling
At the boiling point, the particles within a liquid have enough energy to overcome their attractive forces and become a gas
Condensation
water vapour is converted back to liquid as the water particles lose kinetic energy and slow down
Heat of vapourisation and condensation
energy that must be added to convert exactly 1g of liquid to gas at its boiling point
Phase transitions diagram
Phase diagrams
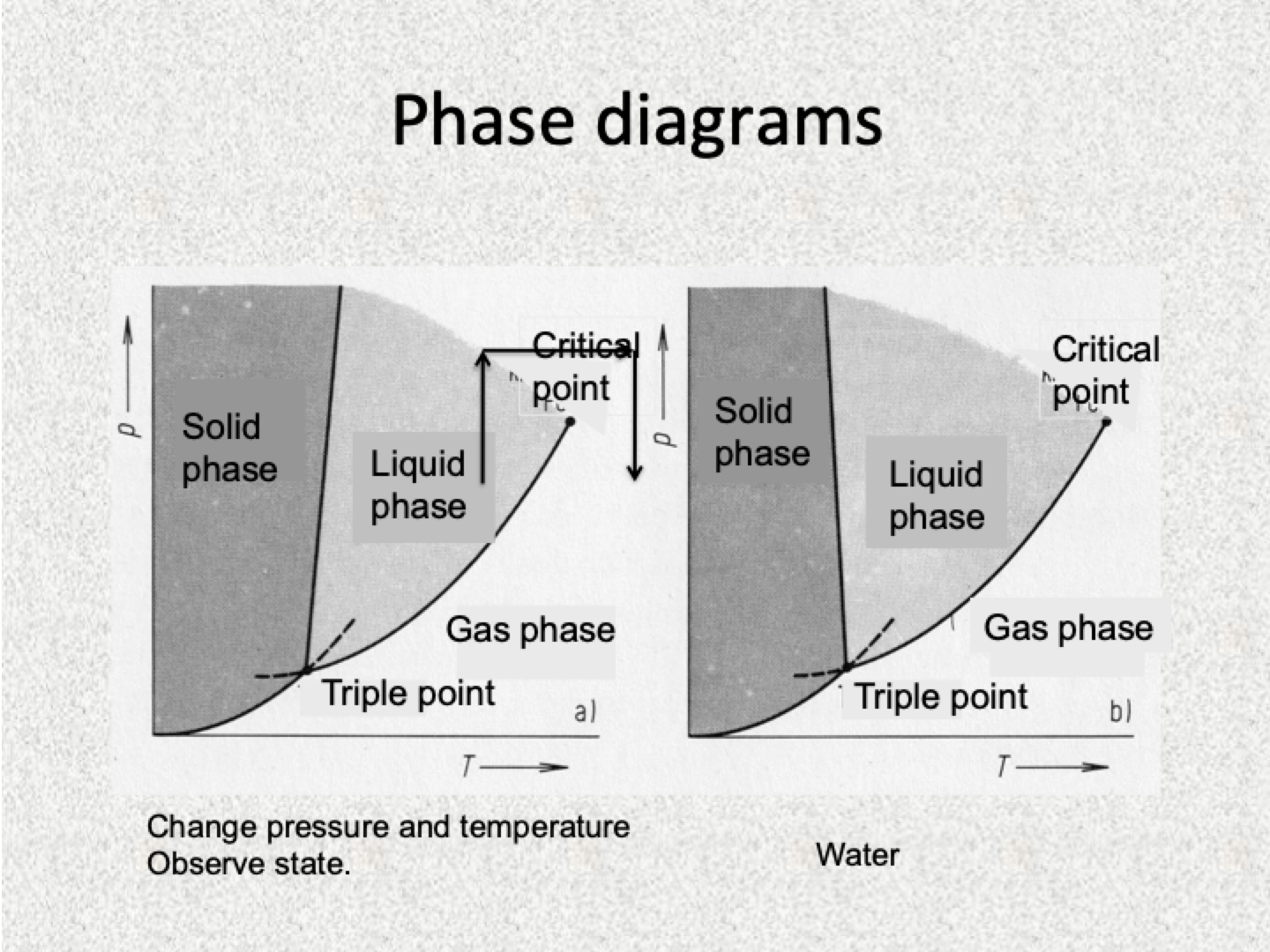
Triple point is fixed and cannot move. It is the place where solid phase, liquid phase and gas phase can exist in equilibrium.
Exothermic reactions
The energy of the products is lower than the energy of the reactants
Heat is released
Negative enthalpy change
Endothermic reactions
The energy of the products is higher than that of the reactants.
Heat is absorbed
Positive enthalpy change
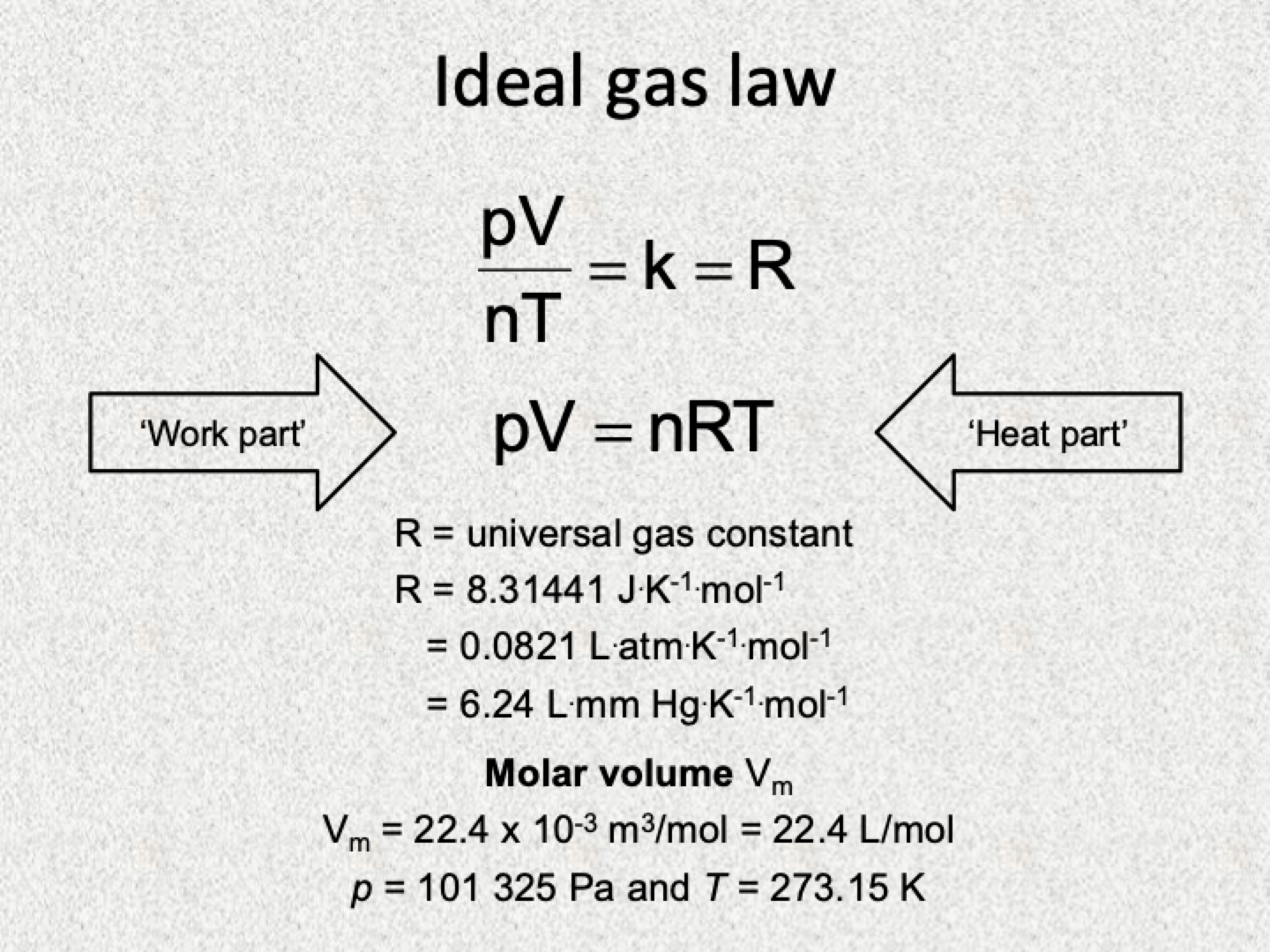
Standard temperature and pressure (STP) conditions
Standard temperature is exactly 0 degrees celsius (273 Kelvin)
Standard pressure is exactly 1atm (760mmHg)
One mole of any gas occupies 22.4L (molar volume)
Ideal gas law
First law of thermodynamics
Energy cannot be created or destroyed, merely changed from one form to another
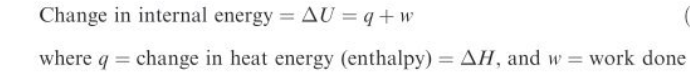
Reaction enthalpy
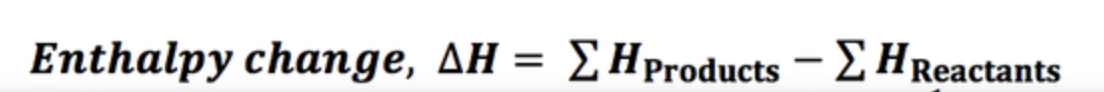
Enthalpy of formation
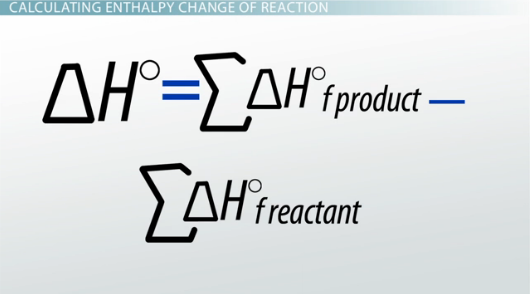
Hess’s Law
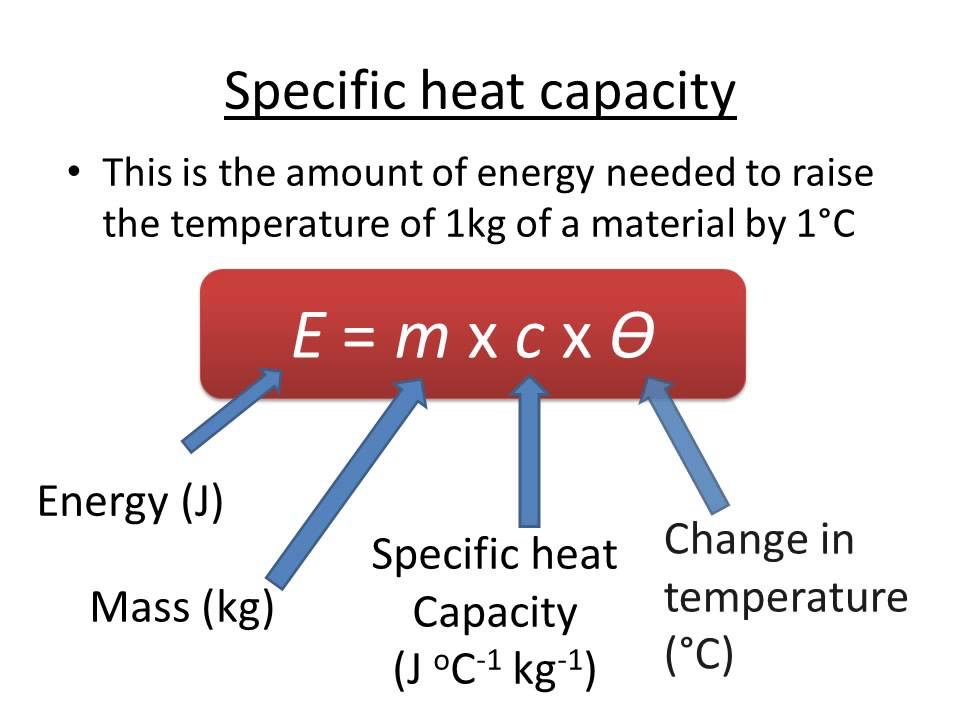
Specific heat capacity
Standard enthalpy change
enthalpy change when all of the reactants and products are in their standard states
Spontaneous process
A spontaneous process is one that is thermodynamically favourable; it occurs naturally without an external driving force. But, the thermodynamics of the process tell us nothing about the rate at which the process occurs.
Non-spontaneous process
A nonspontaneous process will not take place unless it is “driven” by the continual input of energy from an external source.
Entropy
measure of ‘disorder’
the larger the entropy the more ‘disordered’
Second Law of Thermodynamics
Entropy (disorder) increases,
Spontaneous processes move toward greater disorder,
Perfect efficiency is impossible
Energy flows naturally from hot to cold regions
Gibbs free energy equation

Gibb’s free energy values
Spontaneous processes
ΔG = ΔH - TΔS < 0
Exergonic
Non-spontaneous processes
ΔG = ΔH - TΔS > 0
Endergonic
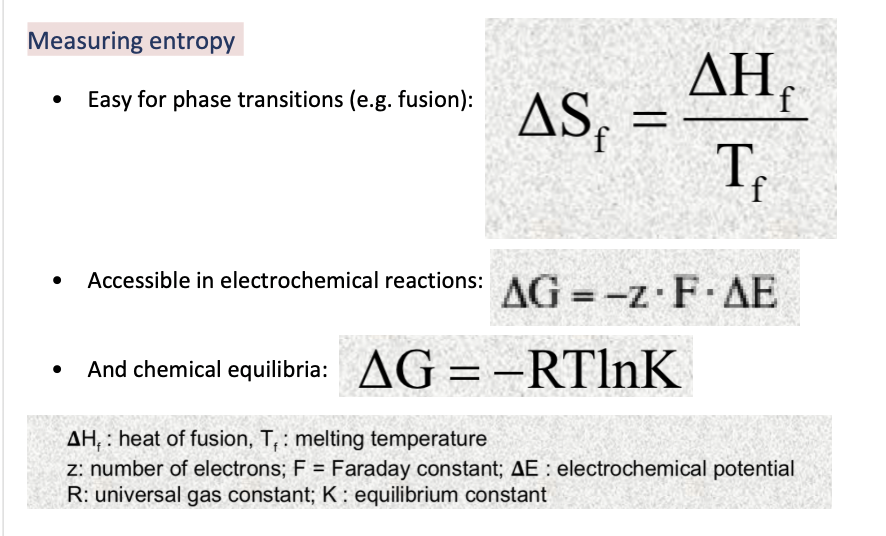
Measuring entropy
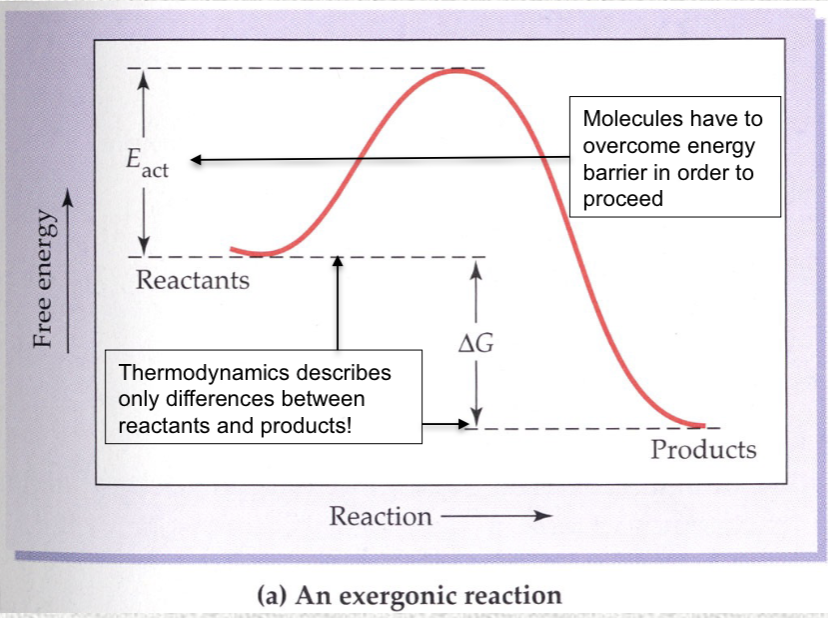
Activation energy
the energy in excess of that possessed by the ground state that is required for the reaction to proceed.
Activation energy in exergonic reaction
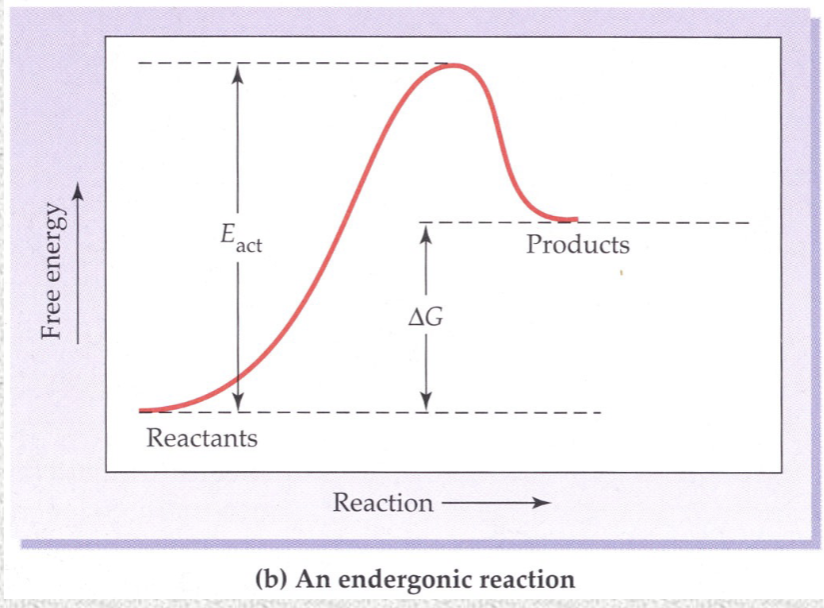
Activation energy in endergonic reaction
Collision theory
1. | The reactants must collide with each other. |
2. | The molecules must have sufficient energy to initiate the reaction (called activation energy). |
3. | The molecules must have the proper orientation. |
Effect of temperature on reaction rate
At higher temps, the increase in kinetic energy of the reactants makes them move faster and collide more often, and it provides more collisions with the required energy of activation
Effect of concentration of reactants on rate of reaction
An increase in concentration of reactants means more collisions between the reactants and the reaction goes faster
Effect of catalysts on rate of reaction
Lowers energy of activation
Acts by providing an alternate pathway with a lower energy requirement
As a result, more collisions form product successfully
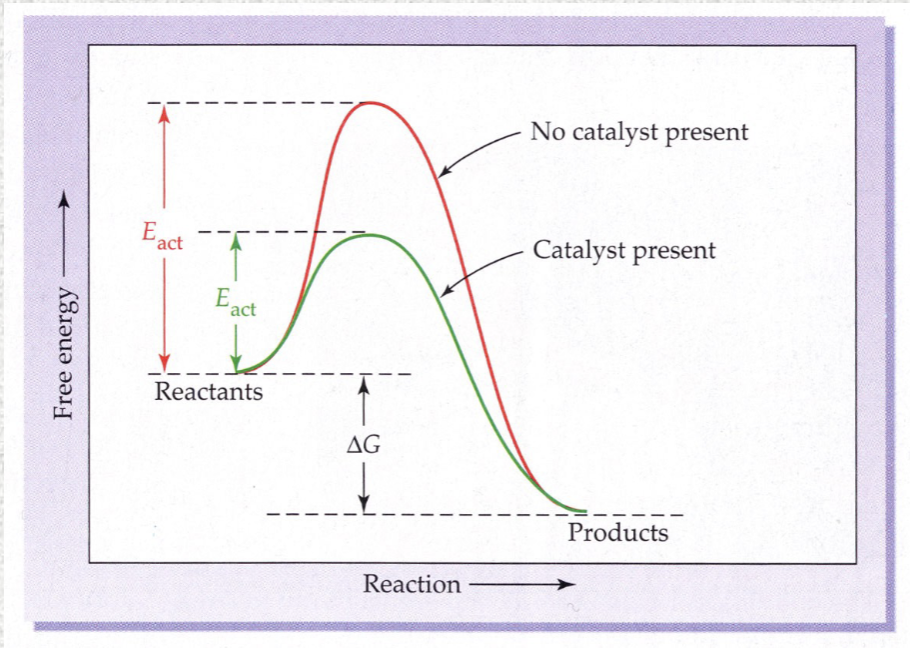
Control of reaction
Activation energy, EA is comparable to energy level at ambient conditions:
Exergonic reactions will happen
Reaction is under thermodynamic control
Activation energy, EA is larger than energy level at ambient conditions:
Exergonic reactions will only happen once EA is provided
Reaction is under kinetic control
Rate of reaction
The change in concentration of a reactant or product with respect to time
Rate equation
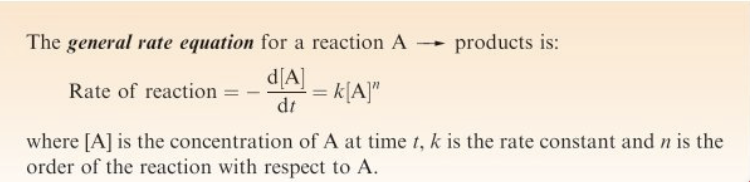
Zero order

First order

Second order

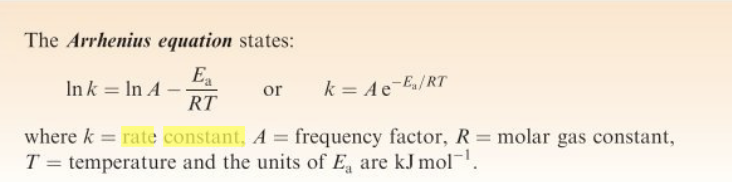
Arhennius equation
k > 1
Exergonic reactions will happen
Reaction is under thermodynamic control
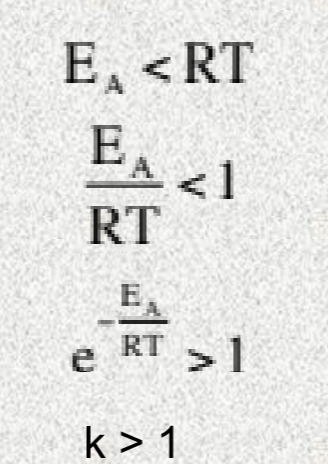
k < 1
Exergonic reactions will only happen once EA is provided
Reaction is under kinetic control

Thermodynamic control
Determines the final state based on energy differences (stability of products).
Kinetic control
Determines the rate of the reaction based on the energy barrier (Eₐ).