LIFE SUPPORT CRAM
1/239
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
240 Terms
Compare cortical and juxtamedullary nephrons
cortical short, peritubular capillaries
juxtamedullary long, peritubular capillaries AND VASA RECTA
Explain loop of Henle
Descending limb: Permeable to water but not solutes; water leaves by osmosis, increasing filtrate concentration.
Ascending limb: Impermeable to water; actively transports Na⁺, K⁺, and Cl⁻ into the medulla, lowering filtrate concentration.
Countercurrent multiplication: Continuous exchange between limbs amplifies the medullary gradient.
Role of macula densa in the nephron
Chemoreceptor for Na and Cl
Influences renin release in RAAS
Surgical management for incontinence
- Botox into bladder
- Urethral bulking
- Male sling (artifical urinary sphincter)
Sympathetic innervation for storage phase
1. Signals sent to sympathetic nuclei in T10-12
2. Signals travel to bladder via hypogastic nerve
3. Relaxes detrusor at B3-AR
4. Contracts IUS (internal urethral sphincter) via A1-AR
What are you looking for when investigating a kidney stone in diagnostic tests (bloods)
Haematuria
Raised WBCs and creatinine in bloods
Symptoms of kidney stones
- Acute severe flank pain
- Nausea
- Urgency
- Testicular pain
Somatic control during storage phase
1. Impulse travels to EUS (external urethral sphincter) via pudendal nerve (roots S2-4)
2. Binds to nicotinic cholinergic receptors on striated muscle -> EUS contracts
This allows filling and storage of urine - rugae (folds in bladder wall) flatten so the walls can distend and increase urine capacity
Causes of kidney stones
- Dehydration
- Too much calcium (hypercalciuria)
- Alkaline urine
- Hyperparathyroidism
- UTI
- Gout (high purine diet of meat and seafood)
Medical management for incontinence
Antimuscarinics to inhibit M3 in urination for detrusor
Beta-3 agonists to promote SNS on storage phase for detrusor
Risk factors for a UTI
GENERALLY: BACTERIA AND URINARY STASIS OR IRRITATION OR URETHRA
- Women have a shorter urethra
- Postmenopausal women have less oestrogen for protection
- Pregnancy has hormonal changes causing urinary stasis
- Elderly have incomplete bladder emptying
- Diabetes mellitus has hyperglycemia which encourages bacterual growth
- Urinary obstruction
- Catheters
- Dehydration means less urine flow and bacteria clearance
- Frequent sexual actvity irritates urethra
- Contraception disrupts vaginal flora
- Poor hygeine means more bacteria
Explain how the voiding phase works under parasympathetic control
1. Afferents from spinal cord to pontine micturition centre
2. When voluntary decision to pee is made, neurones fire to excite the sacral preganglionic neurons
3. Pelvic nerve stimulated (root S2-4)
4. Ach released to work on M3, contracting detrusor
5. Pontine micturition inhibits Onuf's nucleus, decreasing SNS so the IUS relaxes
6. We consciously reduced EUS contraction so there is distension of urethra
7. Urine passes
ANS control on peeing
parasympathetic: pee
sympathetic: store
somatic: choose
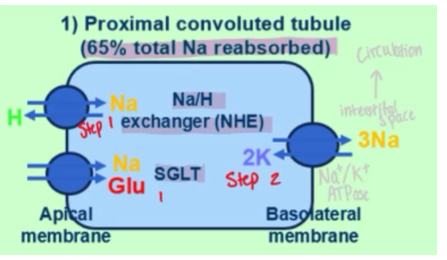
How does the proximal convuluted tubule (PCT) reabsorb glucose
1. a SGLT (sodium-glucose linked transporter) moves sodium and glucose from lumen into the PCT, also NHE which makes sodium enter and hydrogen leave cell
2. this forms a conc gradient of high Na outside and low Na inside. There is a Na/K ATPase that maintains this (as sodium leaves cell, potassium enters)
3. at the basolateral membrane, there is a GLUT transporter that binds to the intracellular glucose and passive diffusion carries it from high intracellular to low interstital (see schematic)
4. glucose then returns to the circulation. anything not reabsorbed will end up in the urine
How does sodium get reabsorbed in the cells of the PCT
SGLT (sodium glucose linked transporter) and NHE (sodium hydrogen exchanger) get sodium pumped INTO the cell
NaK-ATPase gets sodium out of cell and into circulation
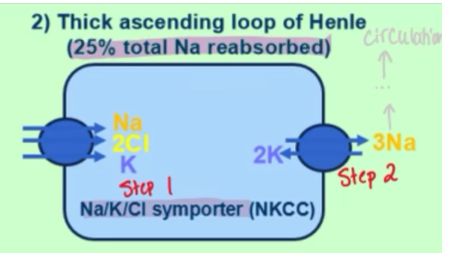
how is sodium reabsorbed on a molecular level in the thick ascending loop of Henle
NKCC (Na K Cl symporter) brings sodium in
NaK-ATPase brings Na out and into circulation
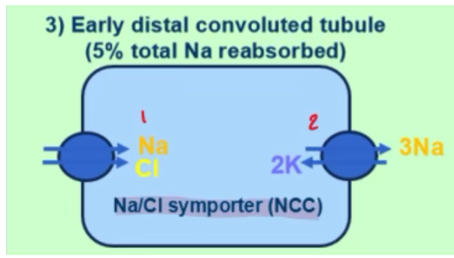
how is sodium reabsorbed on a molecular level in the early distal convoluted tubule (DCT)
NCC (Na Cl symporter) brings Na in
NaK-ATPase brings Na out and into circulation
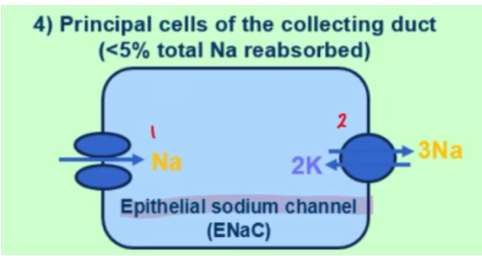
how is sodium reabsorbed on a molecular level in the principal cells of the collecting duct
ENaC (epithelial sodium channel) brings Na in
NaK-ATPase brings Na out and into circulation
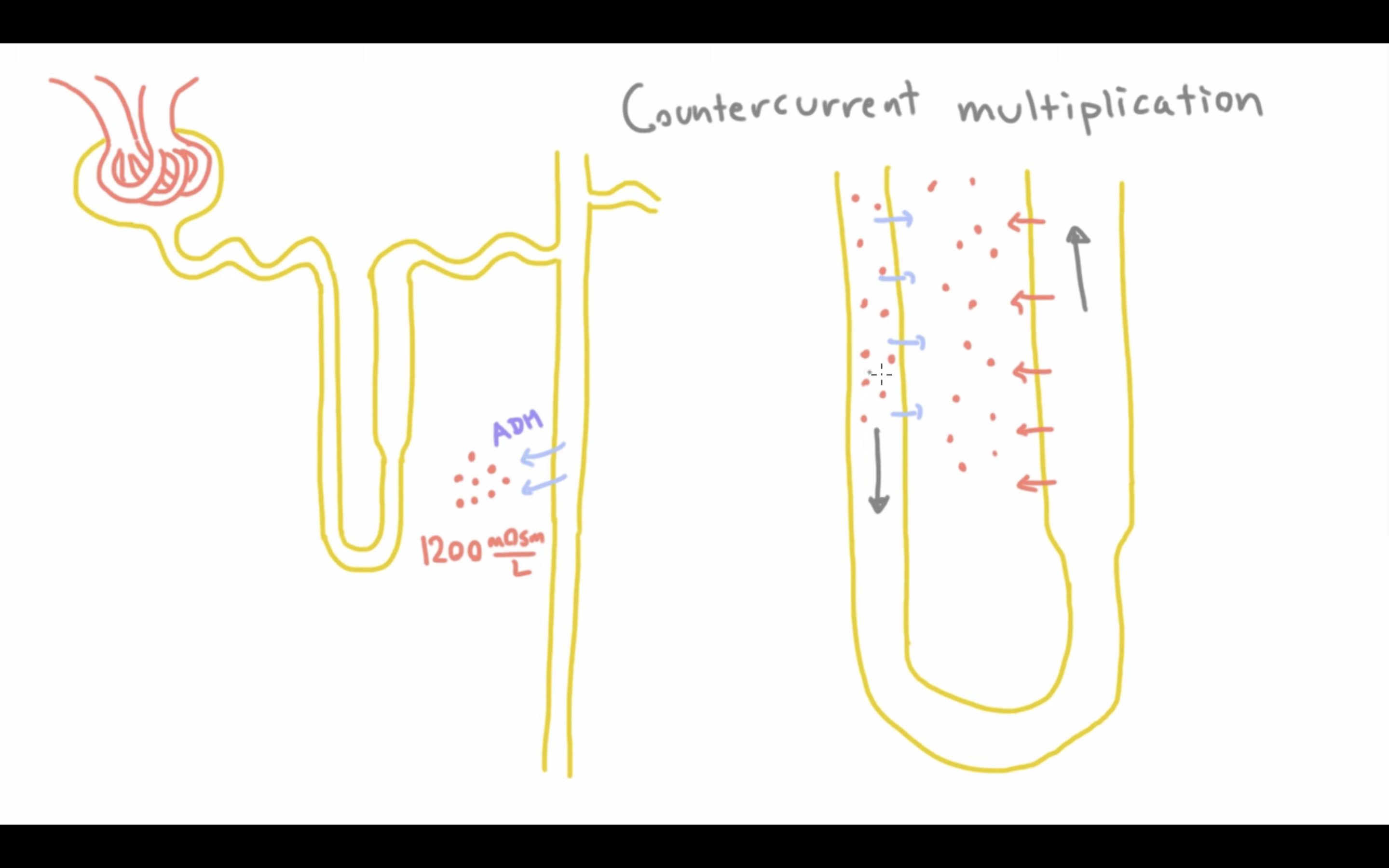
How does counter-current exchange maintain the hyperosmotic gradient FOR THE LOOP OF HENLE TO KEEP DOING ITS COUNTERCURRENT MECHANISM
The thick ascending loop releases NaCl which will be absorbed by the vasa recta (capillaries surrounding LoH)
It will travel to the other side of the vasa recta and then release it into the medulla - this maintains the gradient for the loop of Henle to work
How can GFR be increased via vasodilation and constriction of afferents/efferents
dilate afferents
constrict efferents
Explain autoregulation and myogenic response that controls RBF when there is an dec in blood pressure
Stretch receptors notice drop in blood pressure
Vasodilation to increase blood pressure and thus lower GFR and RBF
What do granular cells secrete, as part of the juxtaglomerular apparatus
renin
equation for GFR
GFR = conc of urine x UFR / conc of plasma
How do macula densa respond to an increased GFR/RBF
There isnt enough time for the NaCl reabsorption
1. Greater NaCl load at macula densa
2. Adenosine release
3. Vasoconstriction of afferent arteriole
4. Lower GFR and RBF
5. MORE TIME FOR NACL REABSORPTION
How can GFR be decreased via vasodilation and constriction of afferents/efferents
constrict affarents
dilate efferents
How do macula densa respond to an decreased GFR/RBF
1. Lower NaCl load sensed by macula densa
2. Release of prostoglandins
3. Vasoconstriction of efferent arterioles
4. Inc hydrostatic pressure in arterioles
5. Greater GFR and RBF
Explain autoregulation and myogenic response that controls RBF when there is an inc in blood pressure
Renal afferent arteriole smooth msucles contain stretch receptors
Stretch receptors sense this
Vasoconstriction means less blood pressure in glomerulus so lower RBF and GFR
Which hormone system is in charge of volume regulation
RAAS
Normal range for ECF osmolarity
285-295
below - dilute
above - conc
Aldosterone secretion can be triggered by RAAS and another ion. Which one?
Increased K+ in plasma
cause of hypernatremia
- commonly, water loss
- gain of sodium (rare)
When given a patient's plasma sodium, how can you calculate their plasma osmolarity
Assuming glucose and blood-urea-nitrogen is normal,
Sodium conc x 2 = plasma osmolarity
Explain hypo-osmotic hyponatremia
'True' hyponatremia
Water excess
Which hormone process is responsible for osmoregulation
ADH
Whats hypernatremia
WATER IMBALANCE
water too low in proportion to the sodium (think of it as a ratio)
Whats pseudo hyponatremia
When another solute is present in a significant enough quantity that reduces the conc of sodium IN RATIO
which makes the sodium appear low
What does aldosterone do to the principal cells lining the collecting duct
Na+ reabsorption (and water with it)
K+ excretion
What does aldosterone do to the intercalated cells lining the collecting duct
H+ and HCO3- get excreted out and this maintains electronegativity which avoids acidosis
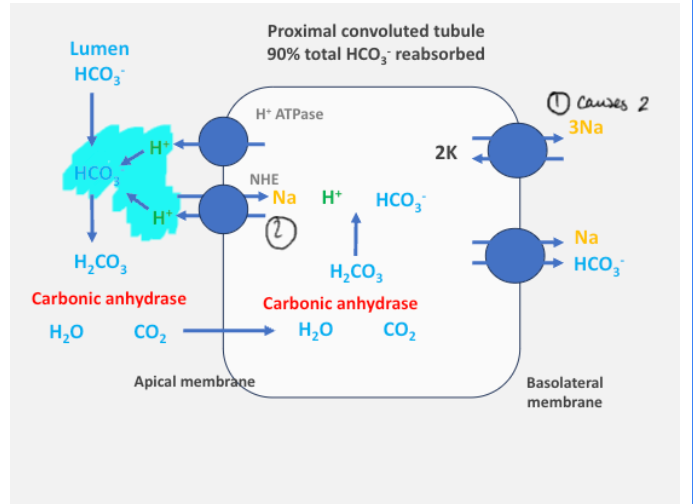
How is the acid-base balance maintained in the PCT
what value determines hyperkalaemia
more than 5.5mM
cause of respiratory alkalosis
dec CO2
cause of metabolic alkalosis
inc HCO3-
cause of metabolic acidosis
dec HCO3-
cause of respiratory acidosis
inc CO2
differentiate between non anion gap and anion gap metabolic acidosis
non anion gap - theres a normal anion gap so the acidosis is due to loss of HCO3- but Cl- will maintain electroneutrality
anion gap - there are other anions that could be due to excess acid, these anions will maintain electroneutrality
what value determines hypokalaemia
less than 3.5mM
Pharmacological causes of hyperkalaemia
ACEi
ARBs
Hypoaldosteronism
Side effects of ACEi and ARBs
Hyperkalaemia (due to RAAS lowering aldosterone)
Lowers GFR
ACEi causes a dry cough due to bradykinin production
pH equation (basically Henderson-Hasselbach simplified)
ABCD
Acidity = Bicarbonate / Carbon Dioxide
How do loop diuretics work
Thick ascending loop of Henle
Inhibits NKCC so lots of electrolytes and water excreted
How do thiazide diuretics work
In the early DCT
Inhibits NCC so lots of electrolytes and water lost
How do K sparing diuretics work
Blocks ENaC in the principal cells of the CD
Increases sodium excretion without K excretion also
Good to be used in combo with loops or thiazides, alone its a weak one
How do vasopressin receptor antagonists work
Inhibits the action of ADH in the collecting duct
Why do loops and thiazides lead to hypokalaemia
Increases ENaC
lots of sodium reaches the CD that would usually be reabsorbed by now
so it gets reabsorbed in the ENaC, meaning K+ gets excreted and gets low
Why are loops good for heart failure patients
Release of PGI2 (prostacyclins) causes vasodilation
How can loops and thiazides lead to metabolic alkalosis
diuretics cause a drop in blood volume
RAAS activates
Ang II stimulates NHE so we lose H+
How does CKD lead to bone mineral disease (BMD)
We gain phosphates from our diet. The GI tract filters it and it gets excreted/reabsorbed in the glomerulus.
CKD has less functioning renal mass so there is phosphate retention.
Phosphate will bind to calcium, forming Ca3(PO4)2
This means we have a lower [free Ca2+] -> calcium deficiency
The parathyroid gland senses this and makes more PTH (parathyroid hormone) in order to increase [Ca2+] (this is secondary hyperparathyroidism)
This calcium comes from the bones (bone resorption - breakdown of bones for mineral release)
Therefore reduced bone mineral density and patients are more prone to pathological fractures
How do we classify CKD
CKD-EPI heatmap
level of proteinuria and eGFR dictate staging
4 indications of renal replacement therapy eligibility
1. Refractory hyperkalaemia
2. Metabolic acidosis
3. Uraemia -> shown clinically via unexplainable weight loss, nausea, itchiness, seizures
4. Refractory pulmonary oedema
Only 1 of these are needed to be eligible
Define Chronic Kidney Disease (CKD)
Abnormality of kidney structure or function that persists for over 3 months
What does albuminuria suggest pathologically
If albumin is leaking into urine, there is a strucutural issue with the podocytes or glomerular basement membrane
How does CKD lead to a vitamin D deficiency?
Kidneys produce alpha-hydroxylase which is needed for activating vitamin D
Less of this enzyme is made so a deficiency occurs
What are some markers of kidney disease
- Albuminuria
- Haematuria
- Electrolyte disorder
- Renal histological abnormalities
- History of kidney transplantation
Why does CKD lead to normochromic-normocytic anaemia
Kidneys produce erythropoietin but with CKD, there are not enough peritubular cells to do so.
Therefore not enough RBCs produced -> anaemia
What are the well-validated formulae for eGFR
MDRD
CKD-EPI (heat map)
Why does nephrotic syndrome lead to hypercoagulability
Protein C and S will be lost in heavy proteinuria
They are important in clotting cascade
Patient will be more prone to clotting and things like DVT or PE
Define nephritic syndrome
An abrupt onset of both haematuria and proteinuria for 1-3 days with a decreased GFR
What value of proteinuria do you need for it to fit the basis for nephrotic syndrome ie be considered heavy
More than 3.5g a day
gold standard scan for a kidney stone
CT with no contrast (CT KUB)
In nephrotic syndrome, you get all 3 of these things. Name them
1. Heavy proteinuria (more than 3.5g a day)
2. Oedema
3. Hypoalbuminaemia (less than 30g/L)
Why does nephrotic syndrome lead to oedema
Hypoalbuminaemia
Less oncotic pressure
So fluid will move to the interstitium (leaky tissues) -> oedema
Normal GFR
90
Investigations for AKI
- Urine analysis for proteinuria, haematuria etc
- ECG for hyperkalaemia
- Bladder scan to identify obstruction
- Bloods for U+E, FBC, liver function, albumin, creatinine, inflammatory markers such as CRP
- CT KUB for stones
Define acute kidney injury (AKI)
Abrupt decline in renal function
What findings in urine analysis are indicative of glomerular disease
- Proteinuria
- Haematuria
- Red cell casts
- Dysmorphorphic RBCs
Whats in rapidly progressive GN
Proteinuria and haematuria for days to weeks
Classifications of AKI
1. Pre-renal: issue with blood vessels (renal artery, vein, afferent, efferent)
2. Renal: nephron issue so filtration issue
3. Post-renal: obstruction of urine flow (ureter, urethra, bladder issue)
Which criteria can stage AKI
KDIGO
Why does nephrotic syndrome lead to hypercholesterolaemia
Hypoalbuminaemia
The liver will try compensate by producing more albumin but it will also produce more lipids
This can lead to patient having high cholesterol
How low does serum albumin need to be for it to count as hypoalbuminaemia under nephrotic syndrome
Less than 30g/L
4 principles of glomerular disease
1. Nephrotic syndrome
2. Nephritic syndrome
3. Isolated proteinuria/haematuria
4. Rapidly progressive GN
ST depression pathology
Ischaemia
Pathology for elevated ST segment
Myocardial infarction
Explain the regulation of iron absorption
Hepcidin inhibits FPN, erythroferrone stops that happening
HIF enhances iron absorbing gene (eg FPN)
Effects on the blood caused by G6PD deficiency
Acute haemolysis
Hb precipitate into Heinz bodies
Which enzyme deficiency in the glycolysis (Embden-Meyerhof) pathway will affect RBC ATP yield
Pyruvate kinase
How does a B12 or folate deficiency affect blood
Megaloblastic (big and immature) anaemia with macroovalocytes (big and oval) and hypersegmented neutrophils
this is due to nuclear maturation
Which parts of the PPP (pentose phosphate pathway) is disrupted by a G6PD deficiency
NADPH and GSH
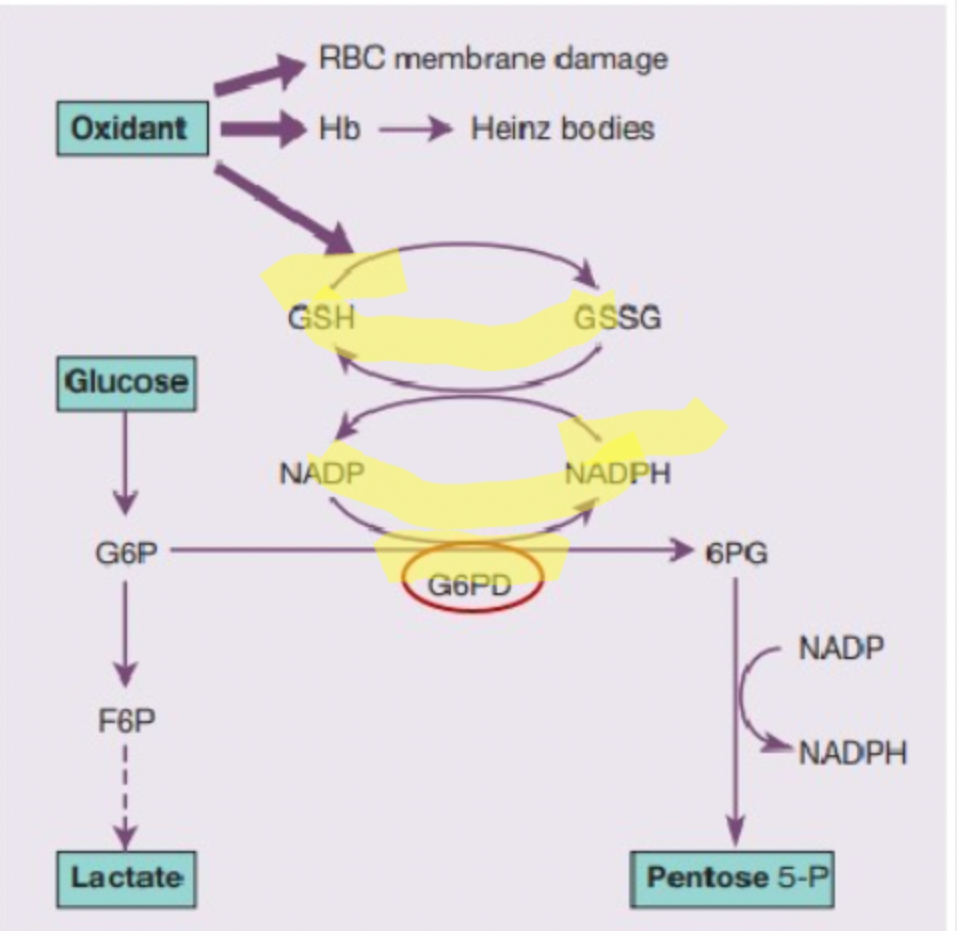
Which enzyme deficiency in the Hexose Monophosphate Shunt/Pentose Phosphate pathway affects RBC's ATP yield
G6PD
How does erythropoiesis generally work
The kidneys detect low oxygen levels in the blood and release erythropoietin (EPO), a hormone that stimulates erythropoiesis.
What is chronic non-spherocytic haemolytic anaemia
RBC break down prematurely
Effects of a pyruvate kinase deficiency for RBCs
- RBC cannot deform shape and then return
- RBC cannot regulate cation conc via Na/K pump --> to maintain the fluid inside a RBC
So cells become dehydrated and rigid from losing the fluid
Chronic non-spherocytic haemolytic anaemia
Within acquired haemolytic anaemia there is immune and non-immune subgroups.
What are the types of immune acquired HA?
1. Autoimmune - immune sustem makes antibodies to attack RBC
2. Alloimmune- a blood transfusion causes antibodies to attack RBC
3. Drug induced - Medications causing haemolysis
How is B12 absorbed
In ileum, bound to IF (intrinsic factor)
Within acquired haemolytic anaemia there is immune and non-immune subgroups.
What are the types of non-immune acquired HA?
1. Red cell fragmentation - physical damage to RBCs on an abnormal surface, there are mechanical or physical forces
2. Infection
3. Physical and chemical agents - drugs or chemical poisoning
The opposite category to acquired HA is hereditary.
Name some haemoglobinpathies in this
- Sickle cell disease (abnormal synthesis of B globin chain)
- Thalassaemias (reduced rate of synthesis of normal alpha and beta globins, they remain in ratio to eachother so if one goes down so will the other)
The opposite category to acquired HA is hereditary.
Name some red cell membrane disorders within this.
- Hereditary spherocytosis (spherical)
- Hereditary elliptocytosis (abnormal shape)
The opposite category to acquired HA is hereditary.
Name some red cell metabolism issues within this, think enzyme deficiencies
- G6PD deficiency
- PK deficiency
What is haemolytic anaemia
Anaemia resulting from an increase in the rate of RBC destruction (haemolysis)