Enthalpy Definitions
1/10
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
11 Terms
Definition for enthalpy change
Enthalpy is a measure of the heat energy in a chemical system. The chemical system can refer to anything such as the atoms, molecules or ions that make up the chemicals.
Definition of activation energy
The minimum energy required to break bonds and start a reaction.
Standard conditions: 25/298k, 101kPa/1atm, 1 mol/dm-3, all species in standard states
Definition of Standard Enthalpy Change of a reaction

The enthalpy change that accompanies a chemical reaction in the molar quantities shown by an equation. This is under standard conditions will all species in standard states.
Definition of Standard Enthalpy change of formation
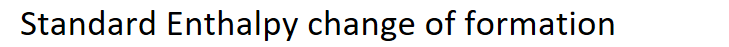
Hf is the enthalpy change that takes place when one mole of a compound is formed from it’s elements. This is under standard conditions with all species in standard states.
Definition of Standard Enthalpy Change of Combustion
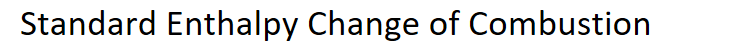
Hc enthalpy change when one mole of a compound reacts completely with oxygen. This is under standard conditions with all species in standard states.
Definition of Standard Enthalpy Change of Neutralisation
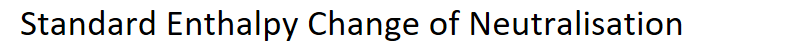
Hneut is the enthalpy change that accompanies the reaction of an acid and a base to form one mole of water.
Definition of Bond energy
Bond Energy is the energy needed to break 1 mole of a particular Bond.
Hess’s Law
The enthalpy change for a chemical reaction is independent of the route taken
Why should you use a Hess cycle
When delta H cannot be determined directly
Bond Energy
Bond Energy is the energy needed to break 1 mole of a particular Bond.
What is enthalpy change