Organic chem unit 30 - isomers
1/19
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
20 Terms
Isomerism
The presence of different compounds with the same molecular formula but different arrangement of atoms
The two types of isomerism are structural and __
stereoisomerism
Stereoisomerism
Different arrangement in space in each isomer of the same atoms that are bonded together in the same order
Cis-trans-isomerism
Caused by restricted rotation around the C=C double bond
Cis isomer
Isomer of a carbon compound containing a C=C double bond with 2 of the same (groups of) atoms on the same side of the double bond
Trans isomer
Isomer of a carbon compound with a C=C double bond where 2 of the same (group of) atoms are on opposite sides of the double bond
Structural isomerism
The same atoms that are bonded together in a different order in each isomer
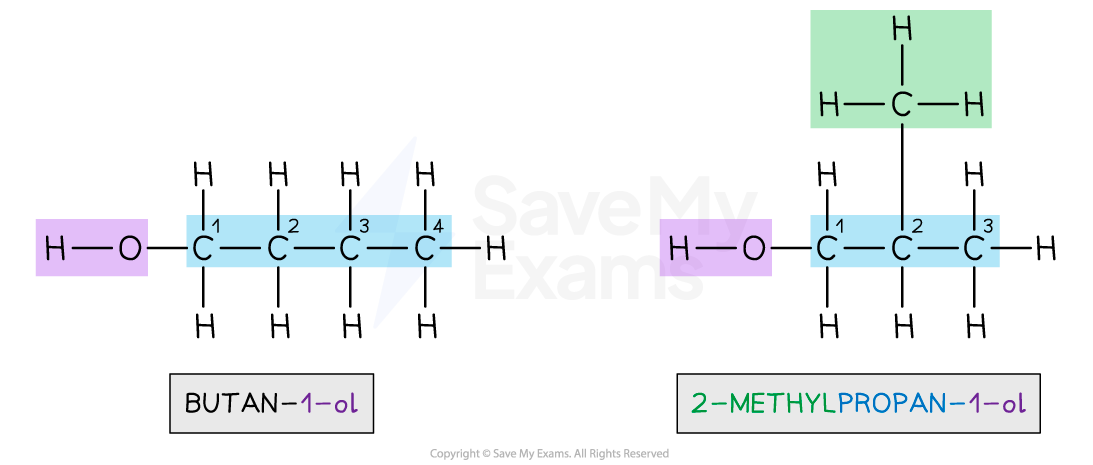
Chain isomers
Carbon compounds with the same molecular formula that differ in lengths of carbon chain due to the possibility of branching
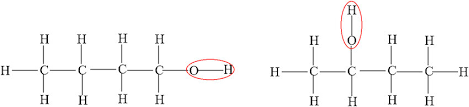
Position isomers
Carbon compounds with the same molecular formula but different positions of the same functional group(s) on the carbon chain
Functional group isomerism
Carbon compounds with the same molecular formula but different functional groups
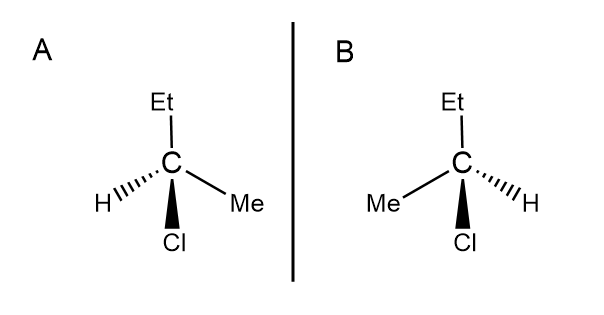
Enantiomerism
Isomers that contain chiral carbon centres bonded to 4 different groups or atoms (in a tetrahedral arrangement)
Chirality
The presence of mirror images of a compound that are non-superimposable upon each other
Plane of polarisation
Optical activity
The ability of a chemical species to rotate a plane-polarised plane of polarised light
Explain the differences in physical properties between butan-1-ol and 2-methylpropan-1-ol in terms of structure.
Explain the differences in physical properties between butan-1-ol and butan-2-ol in terms of structure.
How do the chemical properties of cis-trans isomers differ?
How do the physical properties of cis-trans isomers differ?
Explain the differences in boiling point between cis-but-2-ene and trans-but-2-ene.
Explain the differences in optical activity in these two stereoisomers.