AQA GCSE Chemistry Paper 1
1/291
Earn XP
Description and Tags
This set should (hopefully) contain everything you need to know for AQA GCSE Chemistry Paper 1. Made using the specification and Freesciencelessons on YouTube. This is designed to be used in Term → Definition mode. You can do it the other way around for extra revision, but it will not be framed as a question. Topic 1 - Atomic structure and the periodic table: Cards 1-75 Topic 2 - Bonding, structure, and the properties of matter: Cards 76-161 Topic 3 - Quantitative chemistry: Cards 162-191 Topic 4 - Chemical changes: Cards 192-263 Topic 5 - Energy changes: Cards 264-292 (Topics 6-10 will be in the Paper 2 flashcards when I make them) (Note: You may need to apply concepts from Topics 1-3 in Paper 2.) Cards starting with ᵀ are triple/separate/chemistry only. Cards starting with ᴴ are Higher tier only. (Cards starting with ᵀᴴ are both.) If you want to remove these cards, star all the other cards and then study with starred cards only. Note: Chromatography is covered in Topics 1 and 8. It is included in the Paper 2 flashcards since there is a required practical about chromatography in Topic 8.
Chemistry
Molecular & Ionic Compounds
GCSE Chemistry
AQA
aqa
gcse
chemistry
science
combined
triple
separate
chemistry only
foundation
higher
trilogy
atomic structure and the periodic table
bonding, structure, and the properties of matter
quantitative chemistry
chemical changes
energy changes
a simple model of the atom, symbols, relative atomic mass, electronic charge and isotopes
atoms, elements and compounds
mixtures
the development of the model of the atom
relative electrical charges of subatomic particles
size and mass of atoms
relative atomic mass
electronic structure
the periodic table
development of the periodic table
metals and non-metals
group 0
group 1
group 7
properties of transition metals
comparison with group 1 elements
typical properties
chemical bonds, ionic, covalent and metallic
chemical bonds
ionic bonding
ionic compounds
covalent bonding
metallic bonding
how bonding and structure are related to the properties of matter
the three states of matter
state symbols
properties of ionic compounds
properties of small molecules
polymers
giant covalent structures
properties of metals and alloys
metals as conductors
structure and bonding of carbon
diamond
graphite
graphene and fullerenes
bulk and surface properties of matter including nanoparticles
sizes of particles and their properties
uses of nanoparticles
chemical measurements, conservation of mass and the quantitative interpretation of chemical equations
conservation of mass and balanced chemical equations
relative formula mass
mass changes when a reactant or product is a gas
chemical measurements
use of amount of substance in relation to masses of pure substances
moles
amounts of substances in equations
using moles to balance equations
limiting reactants
concentration of solutions
yield and atom economy of chemical reactions
percentage yield
atom economy
using concentrations of solutions in mol/dm³
use of amount of substance in relation to volumes of gases
reactivity of metals
metal oxides
the reactivity series
extraction of metals and reduction
oxidation and reduction in terms of electrons
reactions of acids
reactions of acids with metals
neutralisation of acids and salt production
soluble salts
the pH scale and neutralisation
titrations
strong and weak acids
electrolysis
the process of electrolysis
electrolysis of molten ionic compounds
using electrolysis to extract metals
electrolysis of aqueous solutions
representation of reactions at electrodes as half equations
exothermic and endothermic reactions
energy transfer during exothermic and endothermic reactions
reaction profiles
the energy change of reactions
chemical cells and fuel cells
cells and batteries
fuel cells
topic 1
topic 2
topic 3
topic 4
topic 5
HT
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
292 Terms
What are all substances made of?
Atoms
What is an atom?
The smallest part of an element that can exist
Approximately how many elements are there?
About 100
What are compounds formed from elements by?
Chemical reactions
What do chemical reactions involve?
Chemical reactions always involve the formation of one or more new substances, and often involve a detectable energy change.
What do compounds contain?
Two or more elements chemically combined in fixed proportions
What is the only way for compounds to be separated into elements?
Chemical reactions
What is a mixture?
A mixture consists of two or more elements or compounds not chemically combined together. The chemical properties of each substance in the mixture are unchanged.
How can mixtures be separated?
By physical processes such as filtration, crystallisation, simple distillation, fractional distillation and chromatography. These physical processes do not involve chemical reactions and no new substances are made.
Describe the process of filtration.
Filtration is used to separate an insoluble solid from a liquid.
Filter paper is placed in a filter funnel, which is placed into a conical flask. The mixture is poured into the filter paper. The liquid will pass through the tiny pores in the filter paper. The solid cannot pass through the filter paper, so it is trapped. The liquid is now separated from the solid.
Describe the process of crystallisation.
Crystallisation is used to separate a soluble solid from a liquid.
If a solution is left for a few days, the liquid will evaporate. This will leave behind crystals of the soluble solid. Crystallisation can happen faster if the solution is gently heated. However, this may cause certain chemicals to break down.
Describe the process of simple distillation.
Simple distillation is used to separate a dissolved solid from a liquid and keep the liquid.
The solution with the liquid and dissolved solid is placed into the flask. The flask is connected to a continuous glass tube, which is surrounded by a jacket called the condenser. Cold water from the tap continuously runs through the condenser, keeping the internal glass tube cold. After running through the condenser, the tap water simply goes down the sink. There is also a thermometer as part of the apparatus.
The solution is heated, for example by using a Bunsen burner. The liquid starts to evaporate, turning into a vapour. The vapour then passes up the glass tube. As the vapour passes over the thermometer, the thermometer reading increases. Usually, the solution is heated until it boils. Next, the vapour passes into the condenser. Because the condenser is cold, the vapour condenses, turning back to a liquid as it passes through the condenser. The liquid is collected in the beaker. At the end, crystals of the solid are left in the flask and the liquid in the beaker.
Describe the process of fractional distillation.
Fractional distillation is used to separate a mixture of different liquids with different boiling points.
The flask contains a mixture of two, or several, different liquids with different boiling points. The flask is attached to a long column containing hundreds of glass beads, called the fractionating column. At the top of the fractionating column, there is a thermometer. Then, there is a condenser like in simple distillation. Cold tap water circulates around the condenser.
The mixture is gently heated. All of the liquids will start to evaporate, but the one with the lower boiling point will evaporate more easily. There is now a mixture of multiple different vapours making their way into the fractionating column. When the vapours reach the fractionating column, they condense and drip back into the flask where they evaporate again. This repeated evaporation and condensation increases the amount of the lower boiling point chemical in the fractionating column. As the warm vapours pass up the column, they reach the thermometer, and the temperature on the thermometer begins to rise, meaning a mixture of multiple different vapours is passing over the thermometer. However, the mixture will contain more of the chemical with the lower boiling point. These vapours now pass into the condenser and turn back into a liquid. However, this liquid is still a mixture of the two chemicals. There comes a point when the temperature on the thermometer stops rising. This will be the lowest of the boiling points. Mainly one chemical is now passing into the condenser. As this chemical condenses, it can be collected in a fresh beaker. This is the first proper fraction. After a while, the temperature on the thermometer begins to rise again. This means that once again, a mixture of vapours is passing into the condenser. However, this mixture mainly contains a chemical with a higher boiling point. When the thermometer reaches a constant temperature, a relatively pure sample of the second chemical is now being collected.
How may new experimental evidence affect a scientific model?
It may lead to it being changed or replaced
What was thought about atoms before the discovery of the electron?
They were thought to be tiny spheres that could not be divided
What led to the plum pudding model of the atom?
The discovery of the electron
What did the plum pudding model of the atom suggest?
That the atom is a ball of positive charge with negative electrons embedded in it
How and why did the new evidence from the alpha particle scattering experiment lead to a change in the atomic model?
Most of the alpha particles passed straight through the gold foil without changing direction. This told the scientists that atoms are mainly empty space. Some alpha particles were deflected. This told the scientists that the centre of the atom must have a positive charge - alpha particles are positive, so any alpha particle that comes close to the positive centre of an atom was repelled and changed direction. Some alpha particles simply bounced straight back off the foil. This told the scientists that the mass of an atom must be concentrated in the nucleus. This nuclear model replaced the plum pudding model.
How was the nuclear model of the atom adapted after the alpha particle scattering experiment?
Niels Bohr suggested that electrons orbit the nucleus at specific distances. The theoretical calculations of Bohr agreed with experimental observations.
Later experiments led to the idea that the positive charge of any nucleus could be subdivided into a whole number of smaller particles, each particle having the same amount of positive charge. The name proton was given to these particles.
The experimental work of James Chadwick provided the evidence to show the existence of neutrons within the nucleus. This was about 20 years after the nucleus became an accepted scientific idea.
What is the relative electrical charge of a proton?
+1
What is the relative electrical charge of a neutron?
0
What is the relative electrical charge of an electron?
-1
How are the number of electrons and the number of protons in an atom related?
They are equal
What is the overall electrical charge of an atom?
Atoms have no overall electrical charge
What is an element’s atomic number?
The number of protons in an atom of that element
How many protons do all atoms of a particular element have?
The same number
How many protons do atoms of different elements have?
Different numbers
What is the radius of an atom?
About 0.1 nm (1 × 10^-10 m)
What is the radius of the nucleus of an atom?
Less than 1/10000 of that of the atom (about 1 × 10^-14 m)
Where is almost all of the mass of an atom?
The nucleus
What is the relative mass of a proton?
1
What is the relative mass of a neutron?
1
What is the relative mass of an electron?
Very small
What is an element’s mass number?
The sum of the protons and neutrons in an atom of that element
What are isotopes?
Atoms of the same element with different numbers of neutrons

What is the mass number and atomic number of this element?
The mass number is 23. The atomic number is 11.
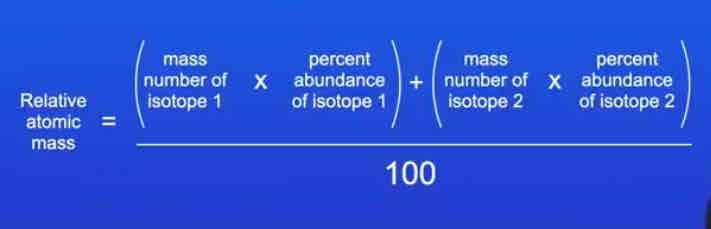
What is the relative atomic mass of an element?
An average value that takes account of the abundance of the isotopes of the element
How is the relative atomic mass of an element calculated, given the percentage abundance of its isotopes?
(This can be extended to include more than 2 isotopes)
What do the electrons in an atom occupy?
The lowest available energy levels (innermost available shells)
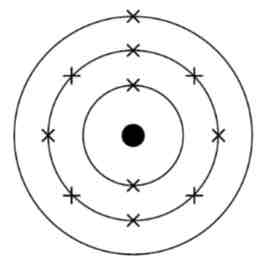
What is the electronic structure of sodium? Use numbers and a diagram.
2, 8, 1
(Note: You should be able to represent the electronic structure of any of the first twenty elements - sodium is the example given in the specification)
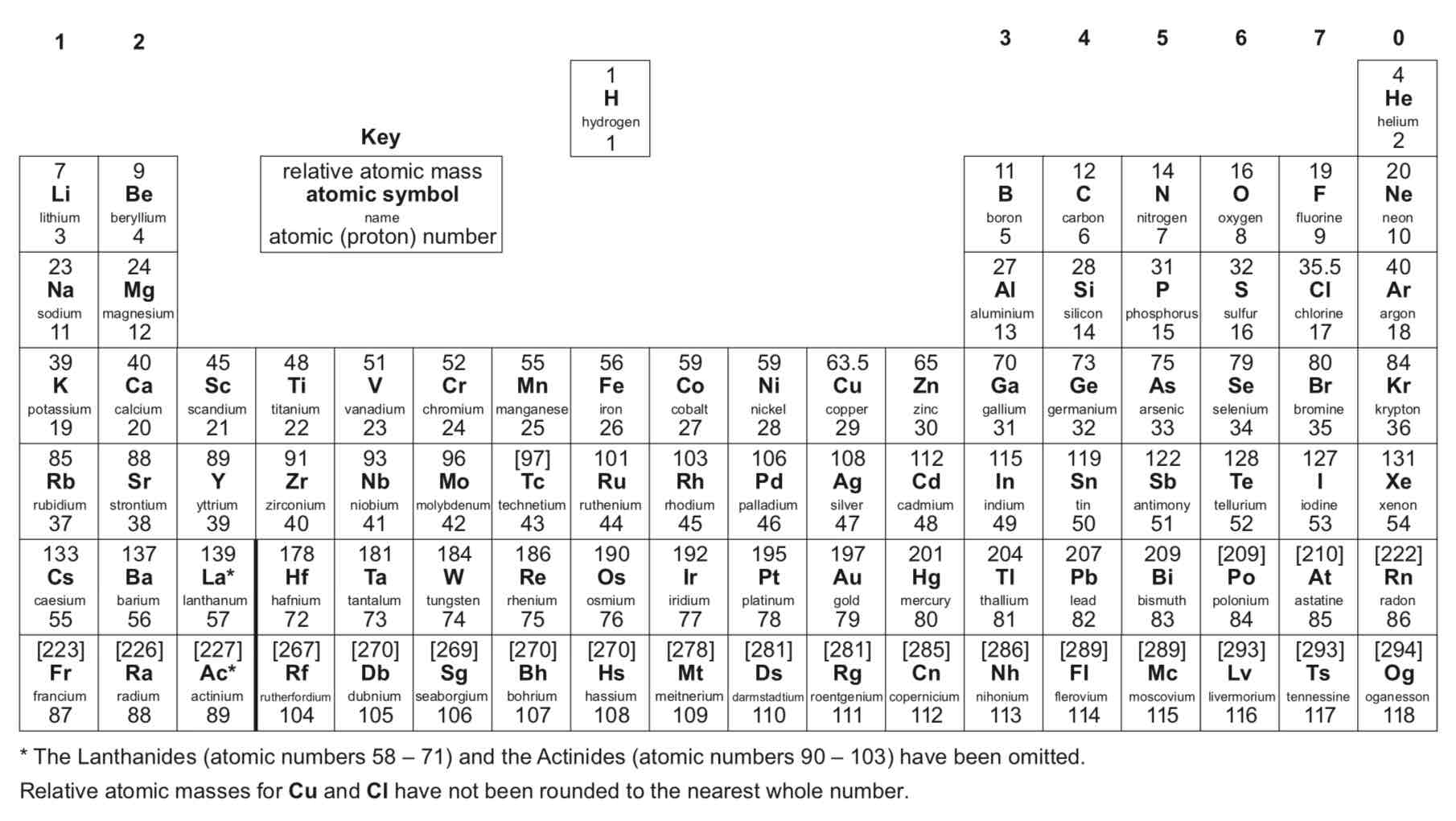
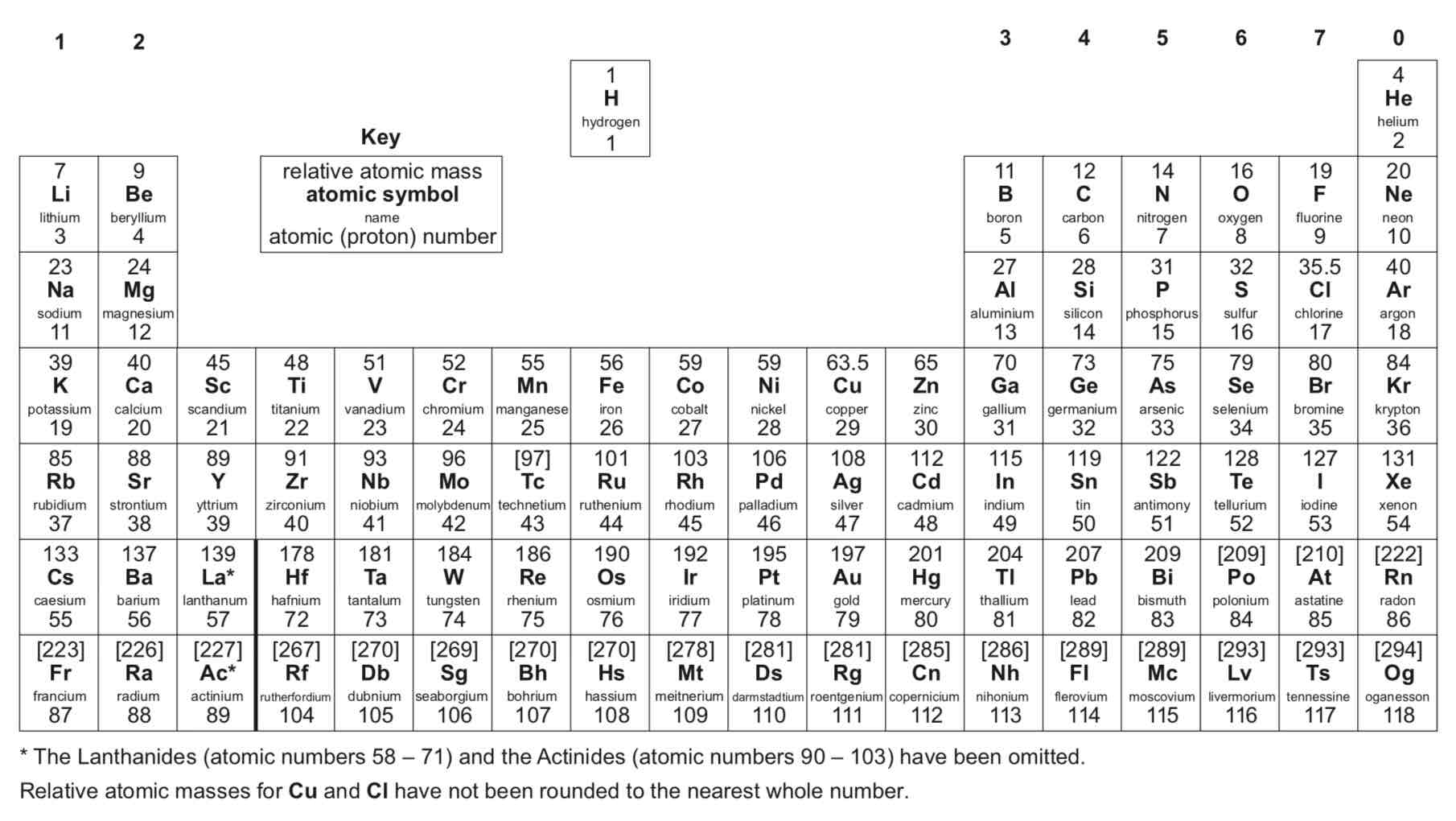
How are elements in the periodic table arranged?
In order of atomic (proton) number and so that elements with similar properties are in columns, known as groups
Why is the periodic table called the periodic table?
Because similar properties occur at regular intervals
What do elements in the same group in the periodic table have? What does this mean?
They have the same number of electrons in their outer shell (outer electrons) and this gives them similar chemical properties
How has the periodic table developed over time?
Before the discovery of protons, neutrons and electrons, scientists attempted to classify the elements by arranging them in order of their atomic weights.
The early periodic tables were incomplete and some elements were placed in inappropriate groups if the strict order of atomic weights was followed.
Mendeleev overcame some of the problems by leaving gaps for elements that he thought had not been discovered and in some places changed the order based on atomic weights.
Elements with properties predicted by Mendeleev were discovered and filled the gaps. Knowledge of isotopes made it possible to explain why the order based on atomic weights was not always correct.
What are metals?
Elements that react to form positive ions
What are non-metals?
Elements that do not form positive ions
Are the majority of elements metals or non-metals?
Metals
Where on the periodic table are metals found?
To the left and towards the bottom
Where on the periodic table are non-metals found?
Towards the right and top
What are the elements in group 0 of the periodic table called?
Noble gases
Why are the noble gases unreactive?
They are unreactive and do not easily form molecules because their atoms have stable arrangements of electrons
How many electrons do the noble gases have in their outer shell?
The noble gases have eight electrons in their outer shell, except for helium, which has only two electrons.
What happens to the boiling points of the noble gases with increasing relative atomic mass (going down the group)?
The boiling points increase
What are the elements in group 1 of the periodic table known as?
The alkali metals
Why do the alkali metals have characteristic properties?
Because of the single electron in their outer shell
Describe the reactions of lithium, sodium and potassium (the first three alkali metals) with oxygen.
They all react rapidly with oxygen, forming lithium oxide, sodium oxide and potassium oxide respectively. Sodium reacts more rapidly than lithium, and potassium reacts more rapidly than sodium.
Describe the reactions of lithium, sodium and potassium (the first three alkali metals) with chlorine.
They all react rapidly with chlorine, forming lithium chloride, sodium chloride and potassium chloride respectively.
Describe the reactions of lithium, sodium and potassium (the first three alkali metals) with water.
Lithium reacts rapidly with water - you can see effervescence (fizzing) - and produces lithium hydroxide (an alkali) and hydrogen gas. Sodium reacts more rapidly with water than lithium and produces sodium hydroxide (an alkali) and hydrogen gas. Potassium reacts with water extremely rapidly, producing potassium hydroxide (an alkali) and hydrogen gas.
Why does the reactivity of group 1 elements increase going down the group?
As you move down group 1, the radius of the atoms increases. This means there is a greater distance between the positive nucleus and the negative outer electron. As this distance increases, the outer electron is less attracted to the positive nucleus. Secondly, the outer electron is repelled by electrons in the internal energy levels. This is called shielding. This decreases the attraction between the nucleus and the outer electron. As you move down group 1, the elements have more electrons in internal energy levels. This means that shielding increases as you move down group 1. For these two reasons, the outer electron is less attracted to the nucleus as you move down group 1. This makes the outer electron easier to lose. So, as you move down group 1, the elements get more reactive.
What are the elements in group 7 of the periodic table known as?
The halogens
Why do the halogens have similar reactions?
They all have seven electrons in their outer shell
Are the halogens metals or non-metals?
Non-metals
What do the halogens consist of?
Molecules made of pairs of atoms
What is the nature of the compounds formed when chlorine, bromine and iodine react with metals?
Ionic
What is the nature of the compounds formed when chlorine, bromine and iodine react with non-metals?
Covalent
What trends are there going down group 7 of the periodic table?
In Group 7, the further down the group an element is the higher its relative molecular mass, melting point and boiling point. The reactivity of the elements decreases going down the group.
Why does the reactivity of group 7 elements decrease going down the group?
When a halogen atom reacts with a metal, the halogen atom gains one electron from the metal atom. In an atom lower down group 7, there is a greater distance between the outer energy level and the nucleus compared to an atom higher up the group. This means that the outer electrons are less attracted to the positive nucleus. Secondly, in an atom lower down the group, there are more electrons in internal energy levels, which repel the outer electrons. This is called shielding. In an atom lower down the group, the level of shielding is greater than in an atom higher up the group. Both increased distance and increased shielding mean that the attraction between the outer electrons and the nucleus is lower on an atom lower down the group than an atom higher up the group. This makes it harder for an atom lower down the group to attract an electron into its outer energy level than for an atom higher up the group. This makes the atom lower down the group less reactive than the atom higher up the group.
What can a more reactive halogen do to a less reactive halogen?
It can displace it from an aqueous solution of its salt
ᵀ What are the transition elements?
Metals with similar properties which are different from those of the elements in Group 1
ᵀ In general, do elements in group 1 or transition elements have higher melting points?
Transition elements
ᵀ Which are more dense: elements in group 1 or transition elements?
Transition elements
ᵀ Which are stronger and harder: elements in group 1 or transition elements?
Transition elements
ᵀ Which are more reactive with oxygen, water and halogens: elements in group 1 or transition elements?
Elements in group 1
ᵀ Which elements have properties that are typical of transition elements?
Chromium (Cr), manganese (Mn), iron (Fe), cobalt (Co), nickel (Ni) and copper (Cu)
ᵀ What are typical properties of transition elements?
Transition elements are typically hard and strong metals, have high melting points, have high densities and are much less reactive than group 1 metals with oxygen, water and halogens. Many transition elements have ions with different charges, form coloured compounds and are useful as catalysts.
What are the three types of strong chemical bonds?
Ionic, covalent and metallic
What particles does ionic bonding occur in?
Compounds formed from metals combined with non-metals
What particles does covalent bonding occur in?
Most non-metallic elements and in compounds of non-metals
What particles does metallic bonding occur in?
Metallic elements and alloys
What happens in ionic bonding?
When a metal atom reacts with a non-metal atom electrons in the outer shell of the metal atom are transferred. Metal atoms lose electrons to become positively charged ions. Non-metal atoms gain electrons to become negatively charged ions. The ions produced by metals in Groups 1 and 2 and by non-metals in Groups 6 and 7 have the electronic structure of a noble gas (Group 0).
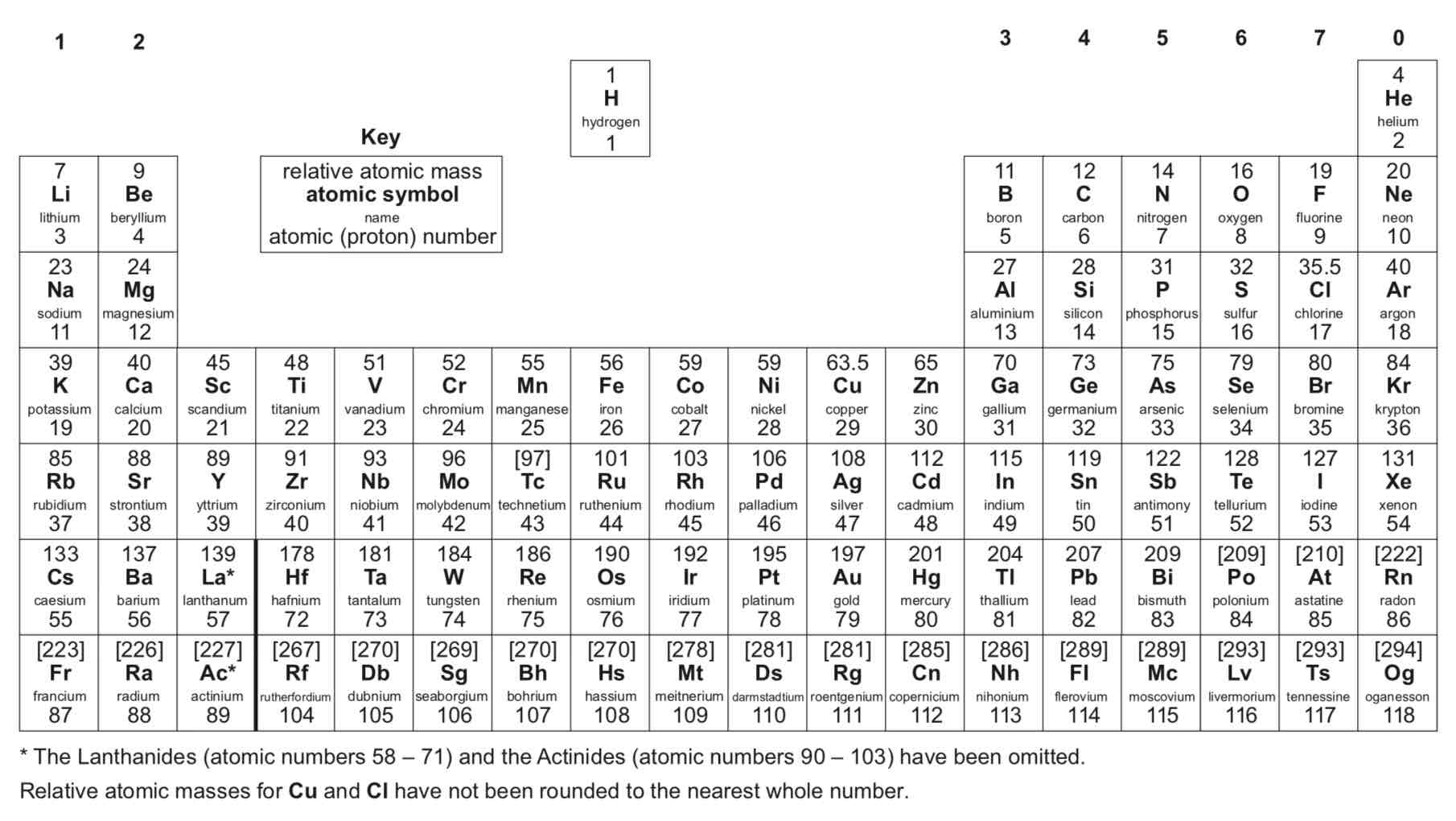
What is the dot and cross diagram for the formation of sodium chloride?
(Note: You should be able to draw dot and cross diagrams for any ionic compounds formed by metals in Groups 1 and 2 with non-metals in Groups 6 and 7 - sodium chloride is the example given in the specification)
What is the charge on an ion of an element in Group 1?
1+
What is the charge on an ion of an element in Group 2?
2+
What is the charge on an ion of an element in Group 6?
2-
What is the charge on an ion of an element in Group 7?
1-
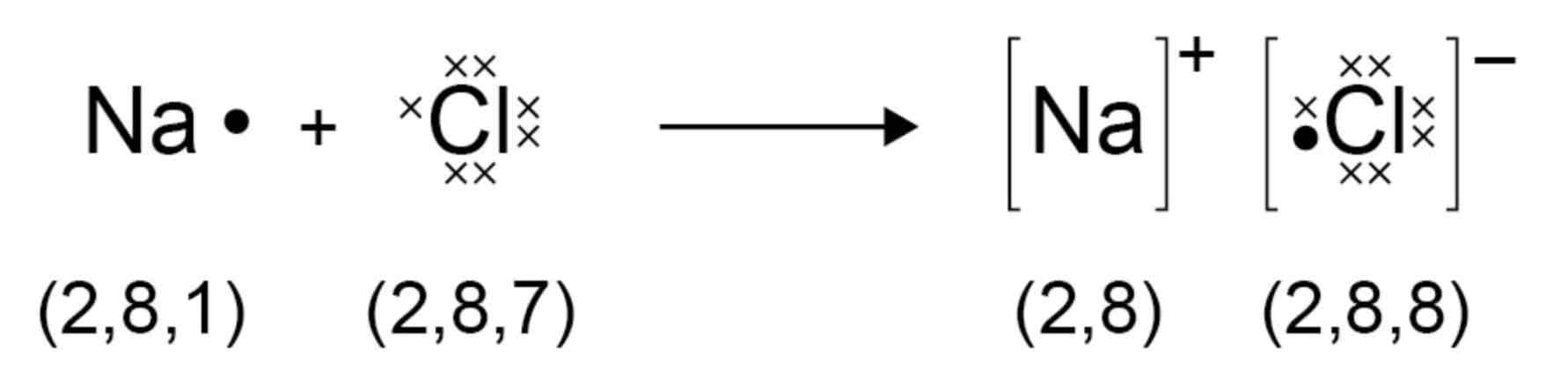
What is the structure of ionic compounds?
An ionic compound is a giant structure of ions. Ionic compounds are held together by strong electrostatic forces of attraction between oppositely charged ions. These forces act in all directions in the lattice and this is called ionic bonding.
How can the structure of sodium chloride be represented?
What are the limitations of using dot and cross diagrams to represent molecules?
Dot and cross diagrams do not tell us about the shape of the molecule.
What are the limitations of using two-dimensional ball and stick diagrams to represent covalent molecules?
Because a covalent bond is shown as a stick, we cannot tell which electron in the covalent bond came from which atom. 2D stick diagrams also give us no idea of outer electrons that are not in bonds, and they do not give us accurate information on the shape of the molecule.
What are the limitations of using three-dimensional ball and stick diagrams to represent covalent molecules?
Because a covalent bond is shown as a stick, we cannot tell which electron in the covalent bond came from which atom. 3D stick diagrams also give us no idea of outer electrons that are not in bonds.
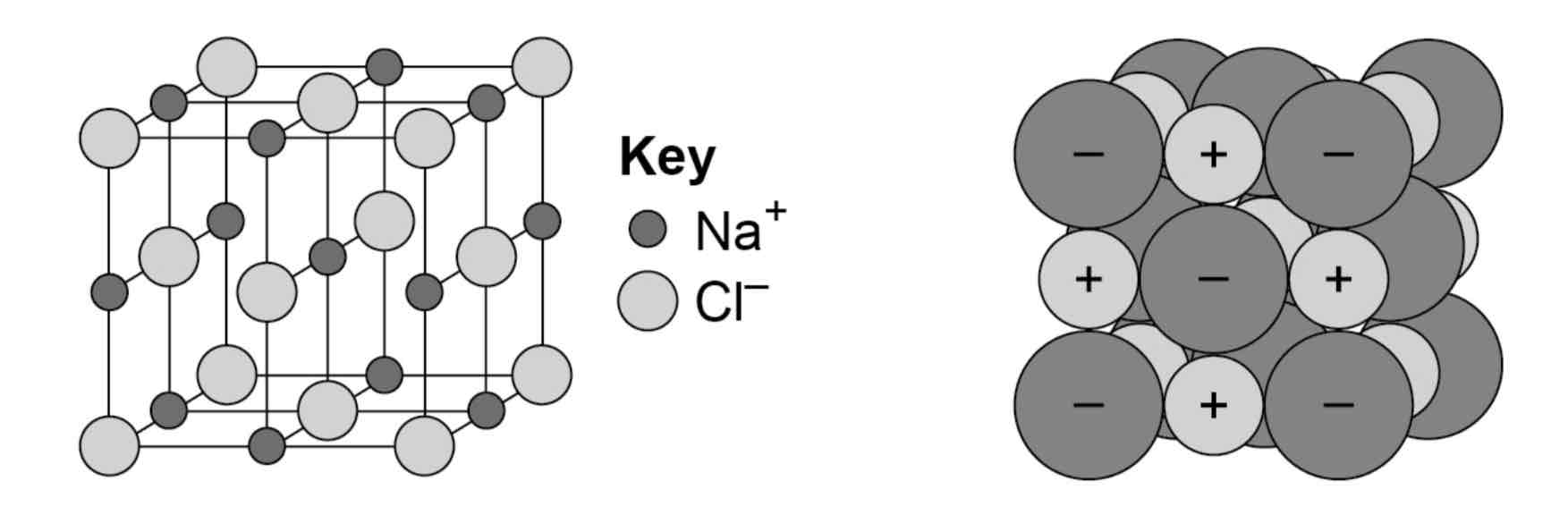
What are the limitations of three-dimensional diagrams to represent giant ionic structures?
In ball and stick diagrams, the ions are shown as widely spaced, when in reality, the ions are packed together.
It can be difficult to see the three-dimensional packing with a space filling diagram.
Both ball and stick and space filling diagrams only show a tiny part of the giant ionic lattice.
What happens when atoms share pairs of electrons?
They form covalent bonds. These bonds between atoms are strong.
What types of covalent structures are there?
Covalently bonded substances may consist of small molecules. Some covalently bonded substances have very large molecules, such as polymers. Some covalently bonded substances have giant covalent structures, such as diamond and silicon dioxide.
How can the covalent bonds in molecules and giant structures be represented?
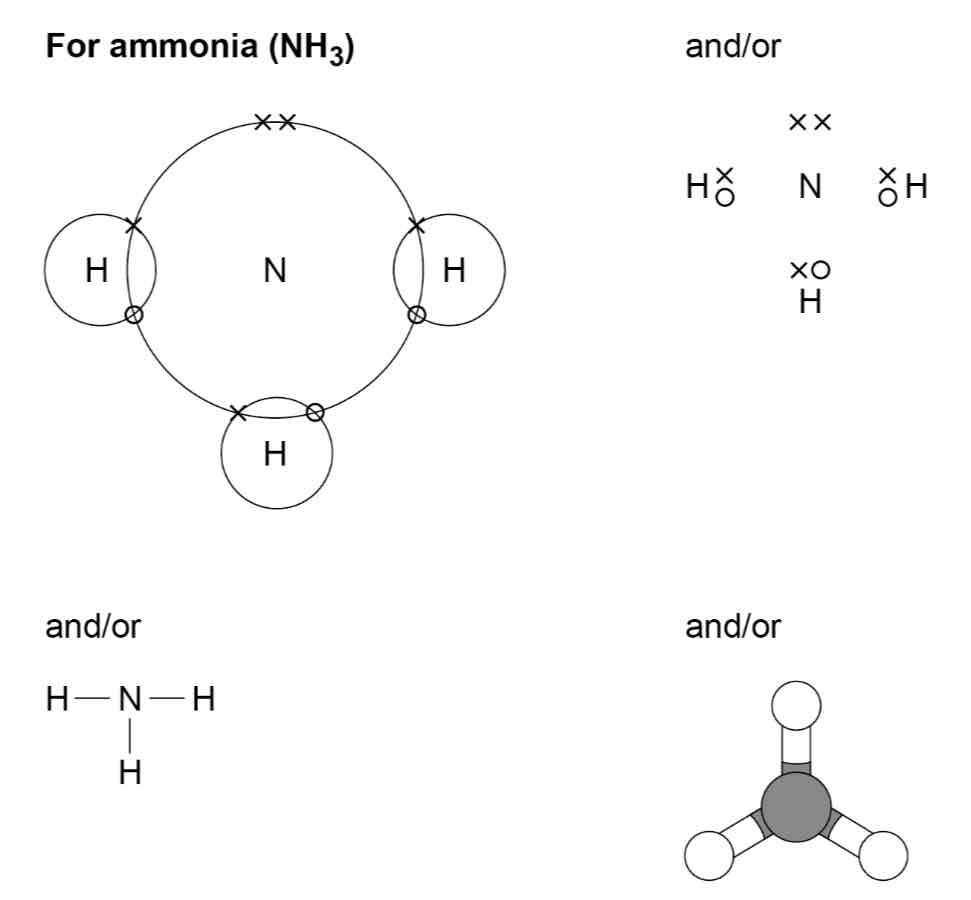
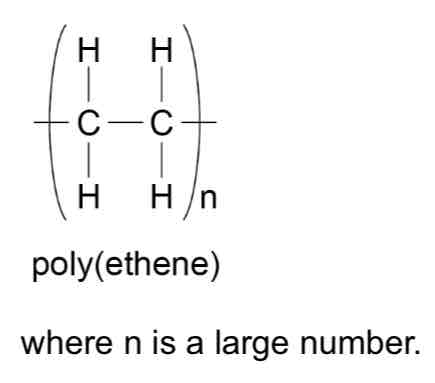
How can polymers be represented?
What is the dot and cross diagram for a molecule of hydrogen?

What is the dot and cross diagram for a molecule of chlorine?

What is the dot and cross diagram for a molecule of hydrogen chloride?

What is the dot and cross diagram for a molecule of water?

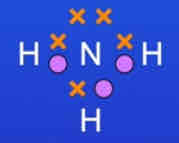
What is the dot and cross diagram for a molecule of ammonia?