HRMS 8th Grade Chemistry Study Guide
5.0(1)
Card Sorting
1/70
Earn XP
Description and Tags
Last updated 2:40 AM on 10/4/22
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
71 Terms
1
New cards
Who arranged the periodic table by atomic mass
Mendeleev
2
New cards
When two or more elements join together chemically
compound
3
New cards
The substance a solute is dissolved into.
solvent
4
New cards
The substance that is dissolved
solute
5
New cards
Particles are evenly distributed throughout giving the same appearance
Solution
6
New cards
What 2 things do living things need to survive
Food and Water
7
New cards
A vertical column on the periodic table with similar properties
Family/Group
8
New cards
What is the electrical charge of proton
positive
9
New cards
What is the electrical charge of electron
negative
10
New cards
What is the electrical charge of neutron
neutral
11
New cards
A row on the periodic table that moves from left to right
Period
12
New cards
Atomic number equals
protons
13
New cards
Protons equal
electrons
14
New cards
Atomic Mass equals
protons + neutrons
15
New cards
Noble Gas have 8 valence electrons so they are
Non-reactive
16
New cards
Most elements are
Metals
17
New cards
Group 1
Alkali Metals
18
New cards
Group 2
Alkaline Earth Metals
19
New cards
Group 3-12
Transition Metals
20
New cards
Group 13
Boron Family
21
New cards
Group 14
Carbon Family
22
New cards
Group 15
Nitrogen Family
23
New cards
Group 16
Oxygen Family
24
New cards
Group 17
Halogens
25
New cards
Group 18
Noble Gases
26
New cards
What does the color of each elements symbol represent
Solid, liquid, gas
27
New cards
How is a elements symbol represented
First letter capital and second letter lowercase
28
New cards
Elements with similar properties are located in the same
Family/Group
29
New cards
Why are the lanthanide and actinides separated
Space requirements
30
New cards
The following elements Boron, Silicon, Arsenic, Germanium, Antimony, Tellurium are
Metalloids
31
New cards
Name the element that makes up 78% of the atmosphere
Nitrogen
32
New cards
Which element does not have a home
Hydrogen
33
New cards
What determines how an element will react with another element
Valance Electrons
34
New cards
That all elements are trying to get a full outer shell of 8
Octet Rule
35
New cards
Name the two elements that are liquids
Mercury and Bromine
36
New cards
The most useful metal in group 13
Aluminum
37
New cards
Which group will the following react group 1
group 17
38
New cards
Which group will the following react group 2
group 16
39
New cards
Group 14 metalloids are used in
Computer Chips
40
New cards
Most reactive metals
Alkali Metals
41
New cards
To the left of the stair step
Metals
42
New cards
To the right of the stair step
Non-Metals
43
New cards
On the stair steps
Metalloids
44
New cards
How is the modern periodic table arranged
Atomic Number
45
New cards
Happens at regular intervals
Periodic
46
New cards
Most Non-Metals are
Gases
47
New cards
What determines what an element is
protons
48
New cards
How many neutrons does chlorine have if its atomic mass is 35 and protons are 17
18
49
New cards
The smallest unit of a substance that keeps all of its properties and chemical characteristics
Atom
50
New cards
What is located in the nucleus of an atom
protons and neutrons
51
New cards
What is located in the energy shells of an atom
electrons
52
New cards
Substances that are not chemically combined
mixture
53
New cards
How many total atoms are represented on the right side of this equation?
6H2O + 6CO2 + sunlight → C6H12O6 + 6O2
6H2O + 6CO2 + sunlight → C6H12O6 + 6O2
36
54
New cards
What is the formula for electron shell configuration
2(n)2
55
New cards
To support the Law of Conservation of Mass How much reactants must be present to the products
same or equal
56
New cards
Explain why helium is special
It is a stable element without having 8 electrons in outer shell
57
New cards
Uniformly distributed particles No difference ex. Milk
Homogeneous Mixture
58
New cards
Unevenly distributed particles you can see the difference ex. pizza
Heterogeneous Mixture
59
New cards
Calculate how many electrons can be in the 5th energy shell
2(5)2 = 2(25) =50
60
New cards
Where are the reactants located
Left side
61
New cards
Where are the products located
Right side
62
New cards
How was the original periodic table organized
Atomic Mass
63
New cards
The universal solvent is
Water
64
New cards
This element will combine with sodium
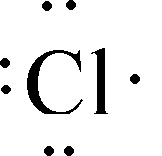
65
New cards
Balance the following equation
4Fe +3O2 Fe2O3
4Fe +3O2 Fe2O3
Add coefficient of 2 on the products side in front of Fe
66
New cards
Balance the following equation
H2 +O2 H2O
H2 +O2 H2O
Add a coefficient in front of H on both reactants and products
67
New cards
The number at the top of a periodic box represents
Atomic number
68
New cards
The Cl represents
Chemical symbol
69
New cards
The Chlorine represents
Element Name
70
New cards
The number at the bottom under element name represents
Atomic mass
71
New cards
Group 17
Halogens