3.2 Environmental Impacts on Enzyme Function
1/8
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
9 Terms
Conditions affecting enzyme activity
Changes in shape(denaturation)
Changes in substrate concentration
Inhibitors
Changes in shape
proteins have a 3D shape held together by different interactions/bonds
if there is a change to the shape of the protein, it will change the function or efficiency of the enzyme
aka denaturation
Denaturation
a loss of both structure and function of an enzyme due to conditions that cause it to unfold from its normal conformation
sometimes can be reversed, but only if the primary structure remains intact and the environment returns to optimal condition before further damage happens
caused by temperature,pH, and chemical environment
temperature-while the rate of the enzyme activity initially increased with temperature increase(due to collision), outside of the optimal range heat can break hydrogen and ionic bonds that hold the protein in the 3D shape
pH- outside of the optimal range, pH can disrupt hydrogen bonds in the enzyme and can alter charges or amino acids, changing the shape of the active site, making it harder or impossible for a substrate to bind
chemical environment-substances around the protein, like salts, detergents, solvents, or alcohol can unfold proteins
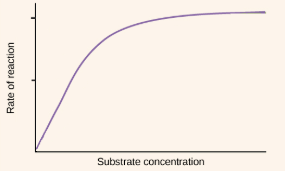
Change in substrate concentration
substrate concentration can change the rate of reactions
at low concentrations, substrate collide infrequently with enzymes which equals a slow reaction rate
as substrate concentration increases, the reaction rate increases (increases collision with enzymes) until the enzymes become saturated(the maximum rate at which it can combine with substrates and produce products)
Regulation of enzyme activity
cells can adjust the activity of enzymes through
competitive and noncompetitive inhibition
allosteric regulation (a form of noncompetitive control)
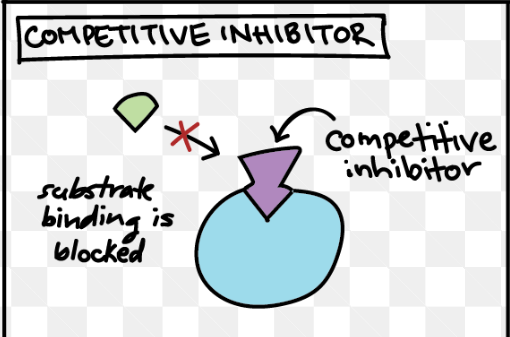
Competitive inhibitors
reduce enzyme activity by blocking substrates from binding to the active site
reversible
inhibition can be reversed with increased substrate concentrations
Noncompetitive inhibitors
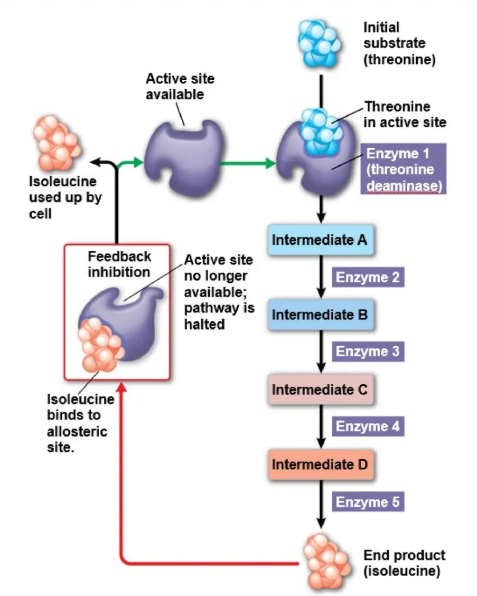
bind to an area other than the active site (the allosteric sites), which changes the shape of the active site, preventing substrates from binding and changing the activity of the enzyme
inhibition can be permanent of reversible
permanent: inhibitor binds with covalent bonds,cell must synthesize new enzymes to overcome effects ex. toxins and poisons
reversible: inhibitor binds with weak interactions, enzyme resumes function when inhibitor releases
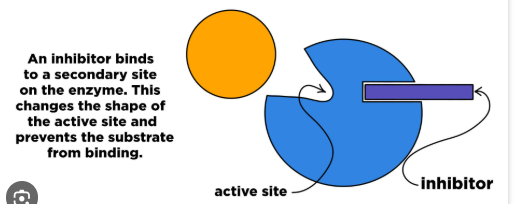
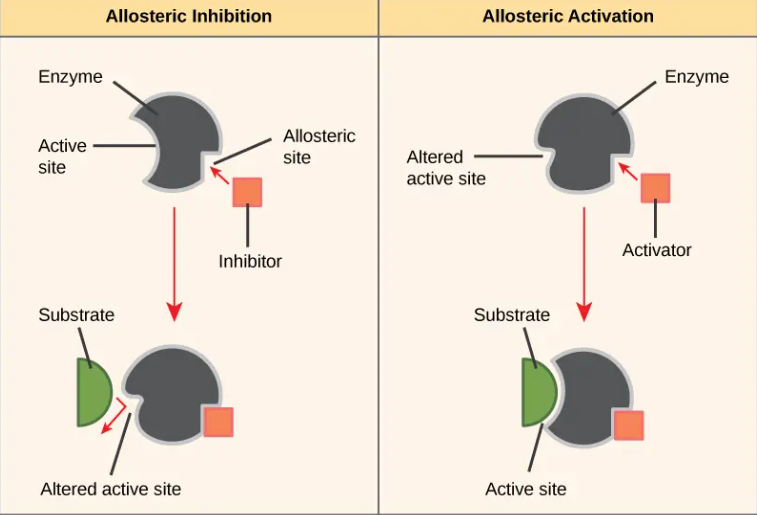
Allosteric regulation
regulatory molecules bind to an allosteric site which either increases enzyme activity(activator) or decreases enzyme acitivity(inhibitor)
a form of noncompetitive control
most noncompetitive inhibitors function through allosteric regulation, but allosteric regulation is a broader category that also includes unique enzymes that have multiple active sites that are regulated allosterically. Allosteric regulation also includes activators
Feedback inhibition
when the end product of a metabolic pathway is an allosteric inhibitor to an earlier enzyme in the same pathway