16 Chemiosmosis
1/39
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
40 Terms
What is a proton gradient?
A concentration gradient of H+ ions across a membrane that stores energy, similar to water behind a hydroelectric dam.
What does H+ ion represent?
A hydrogen atom that has lost its electron, effectively a proton.
What are the alternative names for a proton gradient?
H+ ion gradient, proton gradient, pH gradient, and protonmotive force.
What process uses the proton gradient to generate ATP?
Chemiosmosis, which involves the movement of protons down a concentration gradient.
What is ATP synthase?
A transport protein that catalyzes the synthesis of ATP from ADP and Pi using the energy from proton movement.
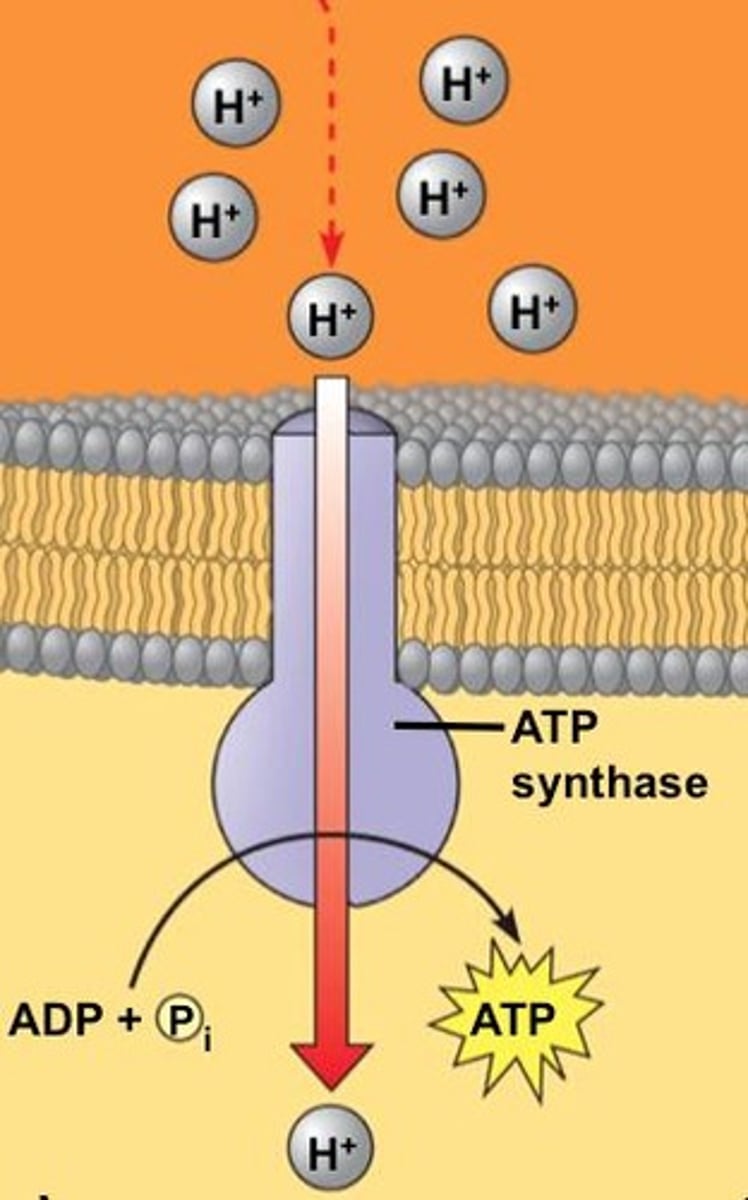
How do protons move through ATP synthase?
Protons pass through two half-channels, turning a rotor that activates the catalytic domain to produce ATP.
Where does the proton gradient form in prokaryotes?
Across the plasma membrane, generating ATP in the cell cytoplasm.
Where does the proton gradient form in eukaryotes?
Across the inner mitochondrial membrane, generating ATP in the mitochondrial matrix.
How is the proton gradient generated?
Through active transport of protons, coupled to exergonic redox reactions.
What is a redox reaction?
A chemical reaction involving the transfer of electrons from an electron donor to an electron acceptor.
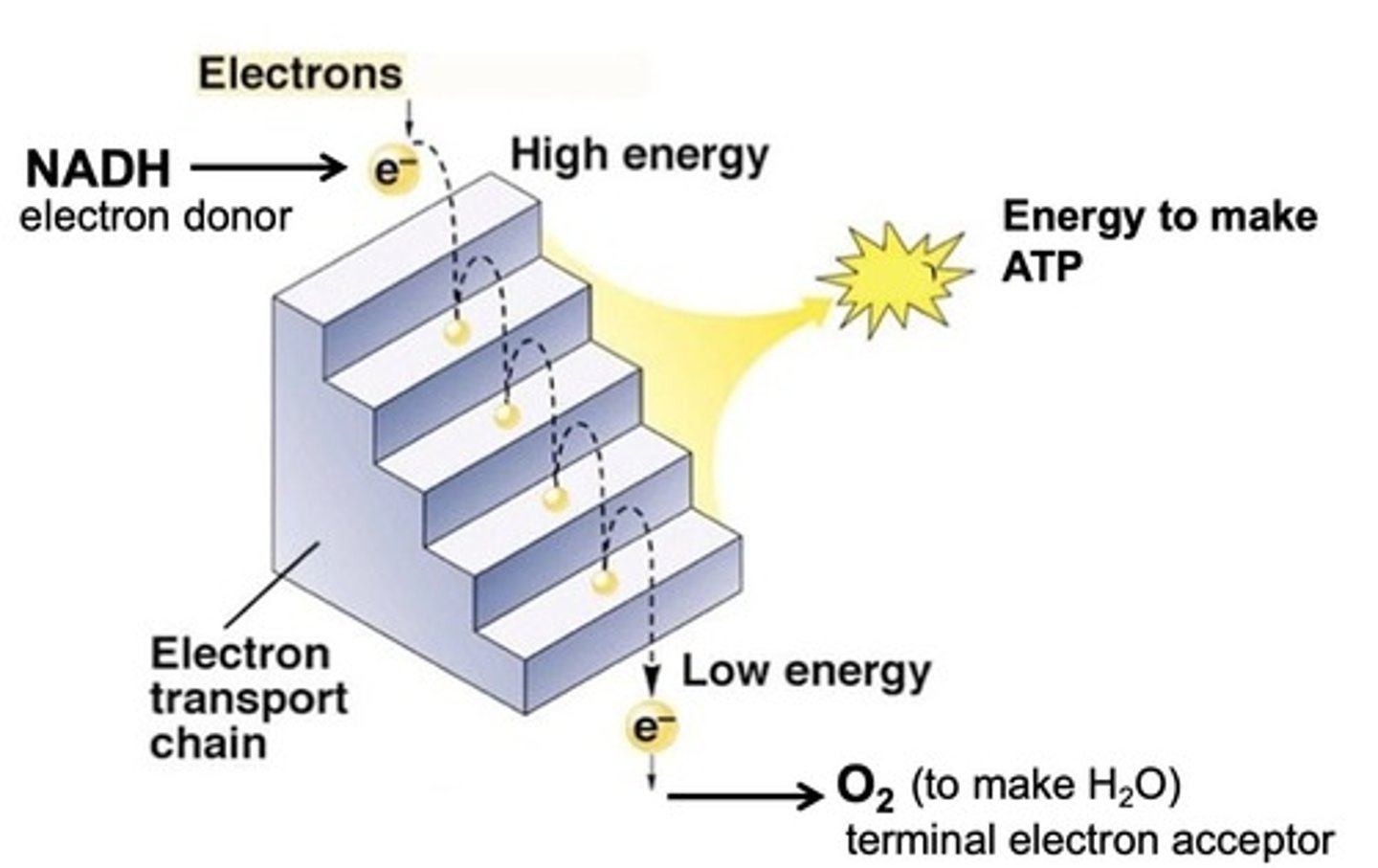
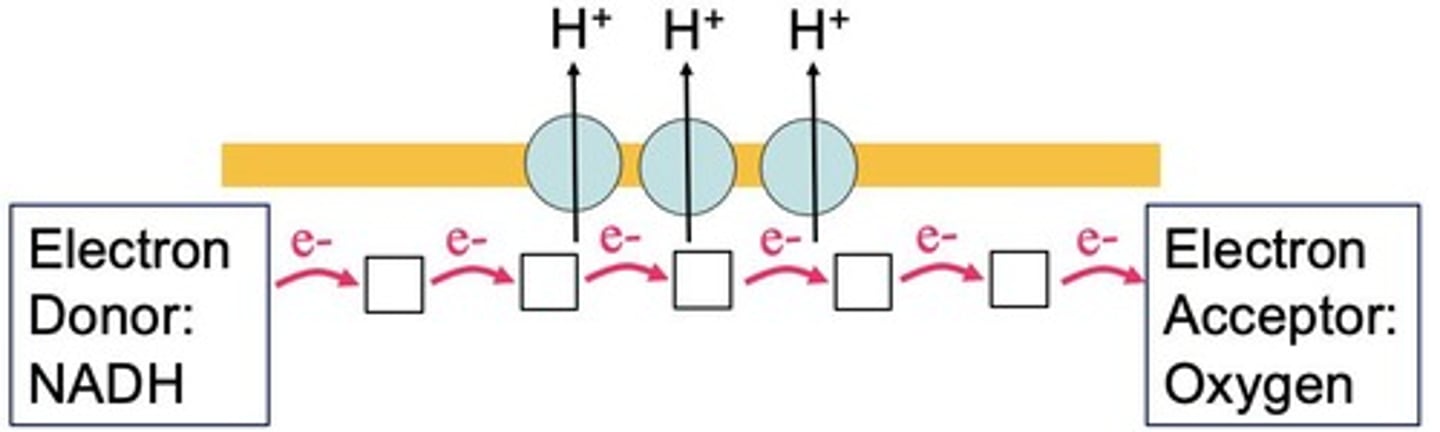
What is the role of the electron transport chain?
To pass electrons through a series of intermediate carriers, releasing energy in small packets to pump protons.
Where are electron transport chains located in prokaryotes?
In the plasma membrane, where protons are pumped out of the cell.
Where are electron transport chains located in eukaryotes?
In the inner mitochondrial membrane, where protons are pumped into the intermembrane space.
What are the electron donors and acceptors in eukaryotes?
NADH (or FADH2) as the electron donor and oxygen (O2) as the terminal electron acceptor.
What is the significance of oxygen in the electron transport chain?
It allows for a large release of energy when electrons are transferred, resulting in more ATP production.
What are the main components of the eukaryotic electron transport chain?
NADH dehydrogenase complex, succinate dehydrogenase complex, cytochrome b-c1 complex, and cytochrome c oxidase complex.
How many H+ ions are transported during electron transport in complexes I, III, and IV?
Each complex transports one H+ ion.
What is the role of intermediate electron carriers in the electron transport chain?
To pass electrons at slightly lower energy levels, allowing for more efficient energy harvesting.
What is the overall function of the electron transport chain?
To create a proton gradient that drives ATP synthesis through chemiosmosis.
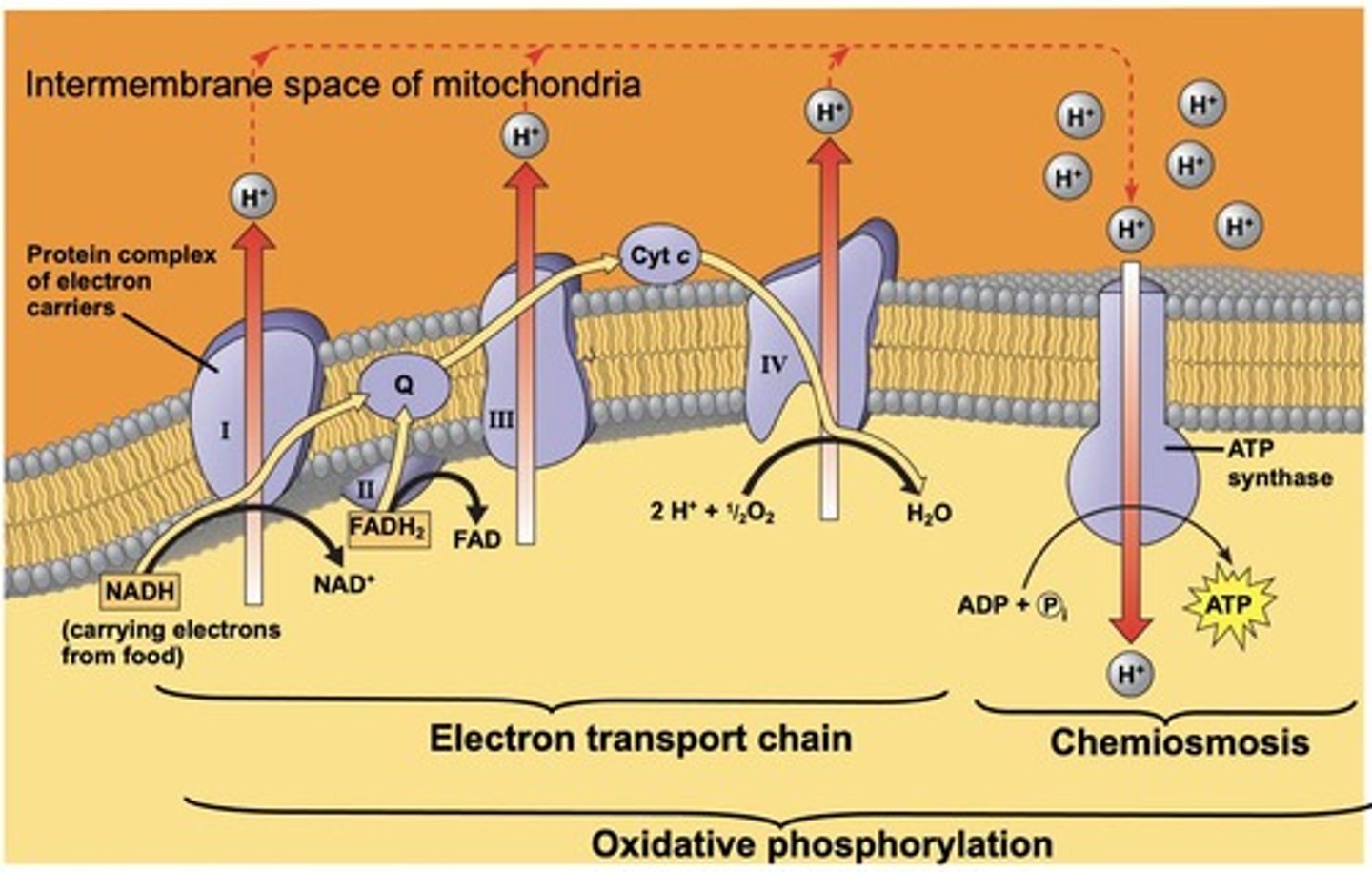
What is the primary function of the electron transport chain in oxidative phosphorylation?
It acts as a proton pump, using energy from electrons to transport protons (H+ ions) across a membrane.
What is created by the energy released from electron transport?
A proton (H+ ion) gradient, which is then used to make ATP by chemiosmosis.
How many ATP are produced from one NADH molecule during oxidative phosphorylation?
Three ATP are produced.
How many ATP are produced from one FADH2 molecule during oxidative phosphorylation?
Two ATP are produced.
What is the role of oxygen in oxidative phosphorylation?
Oxygen serves as the terminal electron acceptor in the electron transport chain.
What happens to the energy of electrons as they pass through the electron transport chain?
The electrons lose energy, which is used to power the active transport of protons.
What are respiratory poisons and give examples?
Respiratory poisons block ATP production; examples include cyanide, carbon monoxide, sodium azide, and rotenone.
What is the function of ATP synthase in oxidative phosphorylation?
ATP synthase synthesizes ATP as protons flow back down their gradient.

What does the acronym OIL RIG stand for in redox reactions?
Oxidation Is Loss, Reduction Is Gain.
What indicates that a substance is being oxidized?
It loses electrons, loses hydrogen, gains oxygen, or increases in oxidation number.
What indicates that a substance is being reduced?
It gains electrons, gains hydrogen, loses oxygen, or decreases in oxidation number.
What is NADH and its role in cellular respiration?
NADH is an electron carrier that transfers high-energy electrons to the electron transport chain.
How are electrons from glycolysis and the citric acid cycle transferred to the electron transport chain?
Electrons are transferred in the form of NADH.
What is the effect of uncouplers like DNP on oxidative phosphorylation?
Uncouplers allow protons to leak through the membrane, dissipating the proton gradient and generating heat instead of ATP.
What is the significance of the evolution of metabolism in relation to proton gradients?
It suggests that life originated in environments with natural proton gradients, which are used for energy production.
What is the relationship between redox reactions and energy efficiency?
Redox reactions are split into smaller steps in the electron transport chain to increase energy coupling efficiency.
What is the structure of NAD?
NAD stands for Nicotinamide adenine dinucleotide.
What happens to protons during chemiosmosis?
Protons flow back down their gradient through ATP synthase to generate ATP.
What is the role of active transport in the creation of a proton gradient?
Active transport moves protons across the membrane, establishing a gradient used for ATP synthesis.
What are electron transport inhibitors and give examples?
Electron transport inhibitors block electron movement in the chain; examples include rotenone and cyanide.
What is the importance of the proton gradient in oxidative phosphorylation?
The proton gradient is essential for driving ATP synthesis through chemiosmosis.