alkanes and alkenes
0.0(0)
0.0(0)
Card Sorting
1/29
Earn XP
Description and Tags
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
30 Terms
1
New cards
why is a carbon- hydrogen bond considered to be relatively non polar
they both have similar electronegativity
2
New cards
what does displayed formula of a compound show?
every atom and every bond in the molecule
3
New cards
when drawing skeletal formula what do the lines represent?
carbon- carbon bonds
4
New cards
when drawing reaction mechanisms what does the curly arrow represent
the movement of a pair of electrons
5
New cards
what is a hydrocarbon?
an organic compound made up of carbon and hydrogen atoms only
6
New cards
what is a homologous series?
a family of organic compounds which have different carbon chain lengths
7
New cards
what is the same between compounds which are part of the same homologous series?
- same general formula
- similar chemical properties
- same functional group
- similar chemical properties
- same functional group
8
New cards
how do compounds part of the same homologous series differ from one another
by ch2 from neighbouring compounds
different physical properties
different physical properties
9
New cards
what is the general formula for an alkane
CnH2n+2
10
New cards
what is the general formula for an alkene?
CnH2n
11
New cards
what is the general formula for a halogenoalkane
CnH2n+1X
12
New cards
what is the general formula for an alcohol
CnH2n+1OH
13
New cards
what is functional group
an atom or group of atoms in an organic molecule which is responsible for the characteristic reactions of that molecule
14
New cards
what is the functional group of an alkane
c-c
15
New cards
what is the functional group of an alkene
c=c
16
New cards
what is the functional group of an alcohol
OH
17
New cards
what is the functional group of an halogenoalkane
the halogen
18
New cards
what is the functional group of an aldehydes
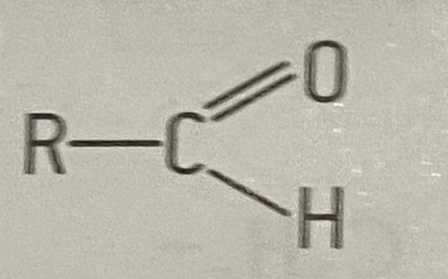
19
New cards
what is the functional group of ketones
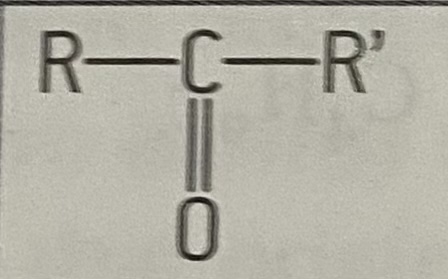
20
New cards
what is the functional group of carboxylic acids
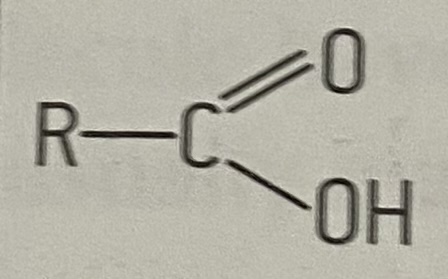
21
New cards
what are the first six roots used in naming organic compounds

22
New cards
what is the suffix of an alkane
-ane
23
New cards
what is the suffix of an alkene
-ene
24
New cards
what is the suffix of a halogenoalkane
none
25
New cards
what is the suffix of an alcohol
-ol
26
New cards
what is the suffix of an aldehyde
-al
27
New cards
what is the suffix of ketones
-one
28
New cards
what is the suffix of a carboxylic acid
- oic acid
29
New cards
how do you name organic compounds (5)
-Compounds are named after the longest carbon chain in the molecule- look at the molecule to find the longest carbon chain. If there is a functional group, it must be the longest carbon chain containing the functional group
- The ending (suffix) identifies the homologous series (functional group)
-The position of branches and the functional group is shown by a number. The lowest possible number is used.
- Numbers are separated by commas
-Numbers and letters are separated by dashes
- The ending (suffix) identifies the homologous series (functional group)
-The position of branches and the functional group is shown by a number. The lowest possible number is used.
- Numbers are separated by commas
-Numbers and letters are separated by dashes
30
New cards
what is structural formula
This shows the unique arrangement of atoms in a molecule in a simplified form, without showing all the bonds.