12-06: Enzymes
0.0(0)
0.0(0)
Card Sorting
1/25
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
26 Terms
1
New cards
biological catalyst
proteins that speed up metabolic reactions by lowering energy barriers
2
New cards
activation energy
minimum energy required to initiate a reaction
3
New cards
substrate
the substance an enzyme acts on and makes more reactive
4
New cards
substrate specific
enzymes depend on the enzymes 3d shape but they are flexible - they won't just bond to anything it has to be their specific substrate
5
New cards
how enzymes work
distort the substrate's chemical bonds so less energy is needed and provides a micro environment more ideal for the chemical rxn. side chains of amino acids can be positioned to participate in the rxn and help with the reactivity (breaks the bonds within the substrates which makes the rxn occur more readily)
6
New cards
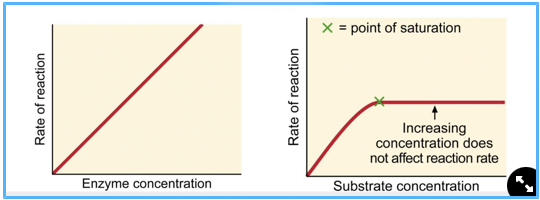
rxn rates, concentration of substrate plays a role in rxn speed
more substrate = faster rxn, UNLESS it becomes saturated, in which case the rxn rate plateaus - if all active sites are occupied.
7
New cards
optimal conditions for enzymes
a cells physical and chemical environment impacts enzyme activity and each enzyme has optimal conditions that it favours (to obtain its most active enzyme conformation, favourite shape). temperature and pH impact the enzyme
8
New cards
optimal temperature for enzymes
allows the greatest # of collisions w/o denaturing the enzyme
e.g. heat makes collisions increase but if there is too much heat, it will denature the enzymes - the optimal temperature is 35-40ºC
e.g. heat makes collisions increase but if there is too much heat, it will denature the enzymes - the optimal temperature is 35-40ºC
9
New cards
optimal pH for enzymes
typically a pH of 6-8 but some will operate at the extremes of pH (like digestive enzymes - e.g. pepsin is lower and trypsin is higher in pH)
10
New cards
cofactors
small non protein helpers for enzymes (e.g. metal atoms of zinc, iron, copper)
some enzymes don't even work in optimal conditions & need help
some enzymes don't even work in optimal conditions & need help
11
New cards
coenzymes
organic molecules (e.g. vitamins)
some enzymes don't even work in optimal conditions & need help
some enzymes don't even work in optimal conditions & need help
12
New cards
inhibitors
molecules that bond to enzymes to make them less effective
13
New cards
competitive inhibitors
molecules that bind reversibly or irreversibly to the active site and compete with the substrate for space in the active site
14
New cards
reversible competitive inhibitors
looks similar and behaves similarly to the substrate, when the competitor is in high concentration to inactivates the enzyme by outcompeting the substrate - reversible as the competitor can be overwhelmed by high concentrations of substrate
15
New cards
irreversible competitive inhibitors
the competitor occupies the active site permanently and therefore deactivate the enzyme, e.g. carbon monoxide, more common
16
New cards
non competitive inhibition
The inhibitor reacts with the allosteric site (some other place on the enzyme that isn’t the active site), changing the shape of its active site
If the shape is altered then the substrate can’t bond to it
If the shape is altered then the substrate can’t bond to it
17
New cards
reversible non competitive inhibitors
used to regulate metabolic pathways in the cell (series of chemical reactions required for a specific product)
18
New cards
irreversible non competitive inhibitors
bind irreversibly wth some other part of the enzyme and permanently denature/inactivate it
Permanently alter the native conformation of the protein
Permanently alter the native conformation of the protein
19
New cards
control of metabolism
Allosteric enzymes have 2 conformations - one catalytically active and the other inactive
Binding of an activator to an allosteric site stabilizes the active conformation
Binding of an inhibitor to an allosteric site stabilizes the inactive conformation
Enzyme activity changes continually in response to changes in the relative proportions of activators and inhibitors
Binding of an activator to an allosteric site stabilizes the active conformation
Binding of an inhibitor to an allosteric site stabilizes the inactive conformation
Enzyme activity changes continually in response to changes in the relative proportions of activators and inhibitors
20
New cards
enzyme saturation
all enzymes are being used, and rxn rate plateaus and if all active sites are occupied
21
New cards
optimal conditions
Each enzyme has optimal environmental conditions that favour the most active enzyme conformation (the enzyme works best when its in its favourite shape and environment)
22
New cards
optimal temperature
allows the greatest number of molecular collisions without denaturing the enzyme - heat makes collisions increase but if there is too much heat, it will denature the enzymes → for most human enzymes is 35ºC - 40ºC
23
New cards
optimal pH
for most enzymes is 6-8 but some will operate at more extremes of pH (like digestive enzymes; pepsin is lower and trypsin is higher in pH)
24
New cards
cofactors
small nonprotein (e.g. metal atoms of zinc, iron, copper) that help the enzymes to function
25
New cards
coenzymes
organic molecules (e.g. vitamins) that help the enzyme to function
26
New cards
inhibitors
molecules that