L2 - The role of carbon in drug molecules
1/38
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
39 Terms
what is a conformation
the spatial arrangement of the atoms affording distinction between stereoisomers which can be interconverted by rotations about formally single bond
what is a configuration
the arrangements of atoms of a molecular entity in space that distinguishes sterroisomers
what can be converted by rotations from single sigma bonds
conformations
what are two forms of conformations
eclipsed and staggered
what is torsional strain
strain that results from eclipsed bonds
what is van der waals strain
strain the results rom atomes being too close together
what is angle strain
strain that results from distortion of bond angles from typical values
formula to calculate stability of saturated cyclic hydrocarbons
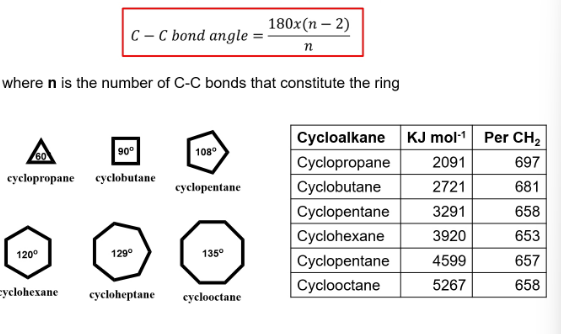
what can affect biological potency
the configuration of chiral molecules
what is the easson and stedman 3 points of interactiojn hypothesis
three points of contact with drug binding site
strong interactions and that drug is active
rotation leads to 1 point of contact
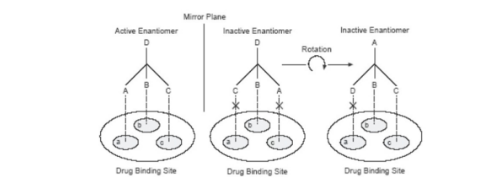
what does rotation lead to in enatomers
only 1 point of contact
why is a double bond important in unsaturated hydrocarbons like z configuration and e configuration
introduces a space that prevents free rotation around that bond
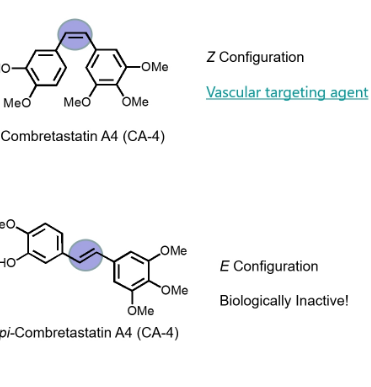
what are wo examples of lipophilic interactions between aromatic rings in drug molecules and biological macromolecules
n stacking and intercalation
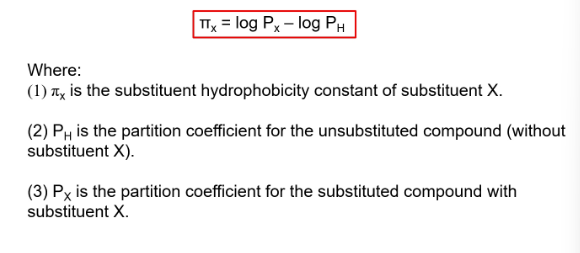
what is the substutuent hydriphobicity constant
a measure of the subsitiuent hydrophobicity compared to hydrogen
basically tells us how hydrophobic or hydrophilic a chemical group (substituent) is compared to hydrogen.
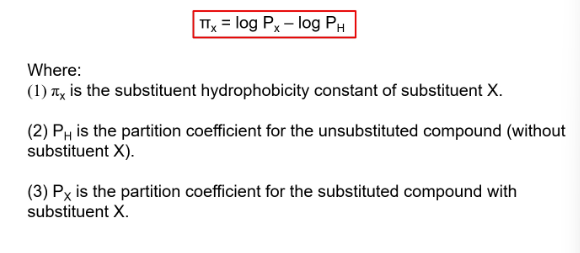
what does positive and negative values imply in the substutuent hydriphobicity constant
positive - more hydrophobic than H
negative - less hydrophobic than H
when pi can be used instead of p
what is the inductive effect in sigma bonds
pushing and pulling of electrons through sigma bonds due to te electronegativitiy of atoms or substiuents attached to a molecules
its like a tug of war
what is the positive inductive effect
when a group pushes away from itself though sigma bonds
what groups are normally in the positive inductive effect
alkyl groups
what is the negative inductive effect
when a group pulls electrons toward itself through sigma bonds
what atoms / groups have a negative inductive effect
those with high electronegativity values such as NO2, CL, COOH
what does the hammet substiuent constant
assesses the electronice effecrs of a substiruent on a benzene ring
what does the mesomeric (resonance) effect refer to
the way electron density is delocalized in a molecule through pi or p bonds.
when does the mesomeric effect take place
when electrons are shifted through bonds in a way that stabilises or dstabilises the molecule
what are the two types of mesomeric effects
+m OR -M
when does the mesomeric +M effect occur
occurs when a group donates electron density through pi bonds
what is the effect from the +M mesomeric effect
it makes the attached atom or molecule more electron rich
what are some examples of +M groups
-OH , -NH2
why is the +M effect more stable and less likely to react
as the groups involved have lone pairs ( like oxygen in OH) that participate in resonance / delocalisation with the pi system donating electrons to it.
when does the -effect occur
when a group withdraws electron density away from the molecule
what is the effec of -M mesomeric
makes attached atom or molecules electron deficient
ecamples of -M groups
NO2, C00H
why is the -M mesomeric effect more reactive and acidic
they participate in delocalisation / resonance by pulling electron density away from the system.
whats the difference between the mesomeric effect and inductive effecr
inductive - happens through sigma bonds, permanent and based on electronegativity
mesomeric effect - happens through pi and p bonds, resonance driven and involves lone pairs or double bonds
what is the hammett subsituent constant σ
number used to measure the electron-withdrawing or electron-donating effect of a substituent group on an aromatic ring.
what does the hammet constant help quantify
the mesometic and inductive effects of substituents.
what does the hammet constant help us predict
the rate of a reaction or the position where a new group will be added on the ring
formula for hammet constant + example picture
σ=log(Khydrogen/ Ksubstituted)
Kₓ is the equilibrium constant (or rate constant) for a reaction with a substituted molecule (substituent X).
Kₓ₀ is the equilibrium constant (or rate constant) for the same reaction with hydrogen as the substituent (i.e., without any substituent on the aromatic ring).
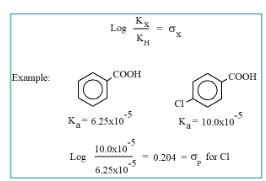
electron withdrawing groups and donating groups postitve or negative σ
withdrawing - positive σ
-donating - negative σ