BIO 1310 Midterm Exam
1/197
Earn XP
Description and Tags
Study Material for Dr. Hale's Midterm
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
198 Terms
Polymers
a series of bonded subunits that form one molecule
a long molecule consisting of many similar or identical building blocks linked by covalent bonds
Monomers
the subunit used to build polymers
the repeating units of a polymer
NOTE: not all subunits are monomers
Dehydration Reactions
the process by which the monomers/molecules are covalently bonded together with the loss of a water molecule
requires at least one hydroxyl group
produces water (removal of water)
Hydrolysis Reaction
the process by which polymers are broken by the addition of water molecule with hydrogen present in one monomer and the hydroxyl group from the other
‘add water’
H20 separates into hydroxyl and hydrogen
Macromolecules
biological polymers, 4 main types
carbohydrates
lipids
proteins
nucleic acids
CARBOHYDRATES
saccharides and their polymers “sugars”
almost all contain a hydroxyl group and always contain carbon with a carbonyl group
MONOSACCHARIDES: Carbohydrate Monomers
Glucose
Fructose
Galactose
DISACCHARIDES: Carbohydrate Dimers
Sucrose
glucose + fructose
Lactose
galactose + glucose
Maltose
glucose x2
ALL are bonded by GLYCOSIDIC LINKAGE
OLIGOSACCHARIDES: Carbohydrate Trimer
Raffinose
galactose + glucose + fructose
Stachyose
glucose + 2galactose + fructose
POLYSACCHARIDES: Carbohydrate Polymers
Main Ones:
Starch (alpha glucose polymer)
many glucose monomers bonded by 1-4 glycosidic linkages
Cellulose (beta glucose polymer)
many glucose monomers bonded by 1-4 glycosidic linkages
has an alternating orientation
produce “sheets” as parallel cellulose strands connect by hydrogen bonds
ALL are bonded by GLYCOSIDIC LINKAGE
Glycosidic Linkage
a covalent bond formed between two monosaccharides by dehydration reaction
a 1-4 bond is between carbon 1 of one monomer and carbon 4 of the other monomer
Functions of Saccharides
storage of sugars/energy
glycogen and starch
structural support
cellulose and chitin
How to Categorize Monosaccharides
by number of carbons in backbone
by location of carbonyl group
Number of Carbon Categories
triose sugars = 3 carbons on backbone
tetrose sugars = 4 carbons on backbone
pentose sugars = 5 carbons on backbone
hexose sugars = 6 carbons on backbone
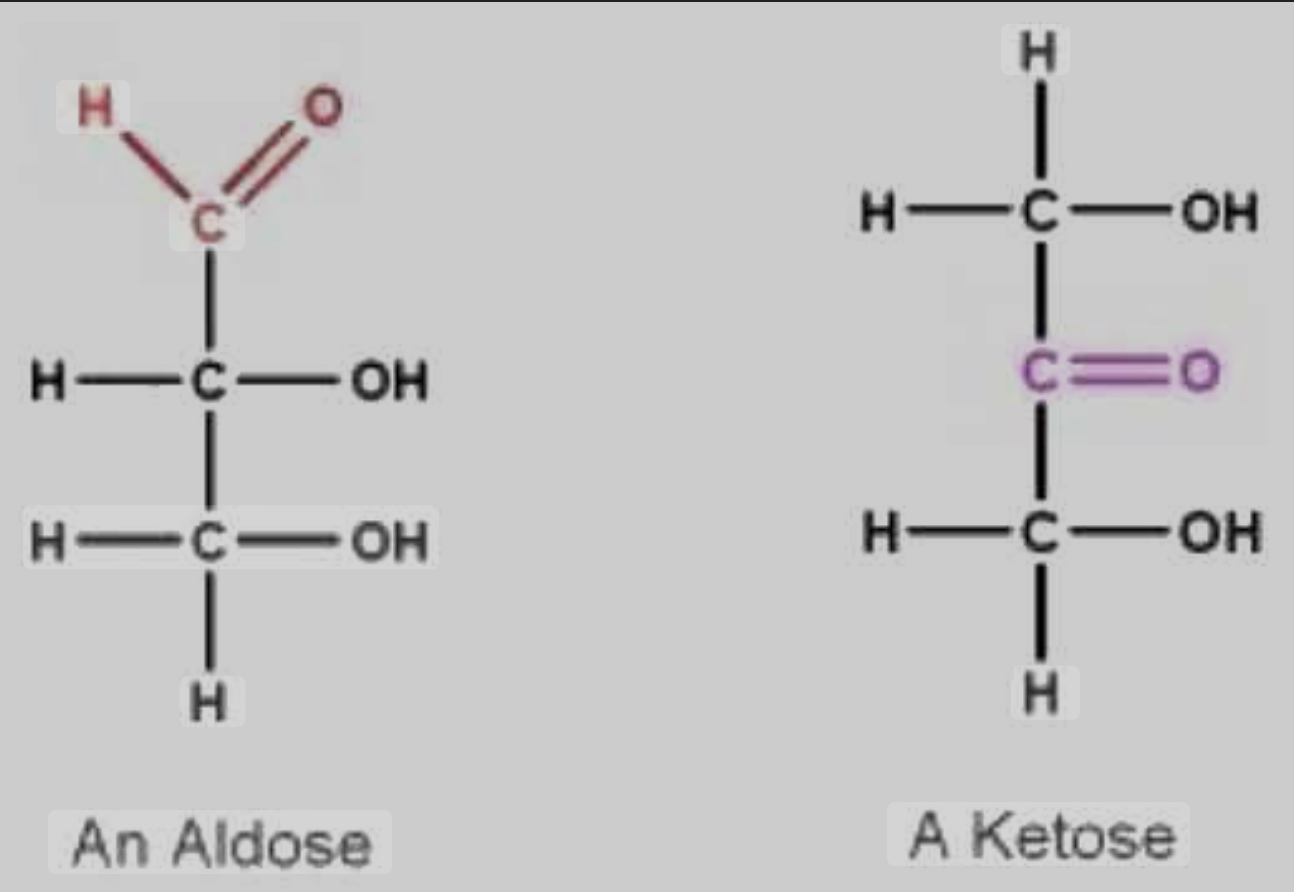
Location of Carbonyl Group Categories
ALDOSE SUGARS (alderhyde sugar)
carbonyl is on terminal carbon
‘alderhyde position’
KETOSE SUGARS (ketone sugar)
carbonyl is on interior carbon
‘ketone position’
Glucose
the principle product of photosynthesis
via CO2 breakdown and carbon grouping and bonding
energy from the sun is stored in non-polar covalent bonds between carbon and hydrogen
Glucose Linear and Ring Form
synthesized linearly but modified into ring form
ring form is more stable in water
changes polarity slightly
Glucose Ring Structure
created by bonding between carbon #1 and the terminating OH-
partial positive charge of C bonds to partial negative charge of O
Process referred to NUCLEOPHILIC ATTACK
Carbons retain linear assigned carbon numbers
ALPHA & BETA Glucose
only difference is the location of the hydroxyl group on carbon #1
cis-trans isomers
generally animals can only produce alpha in large quanitities
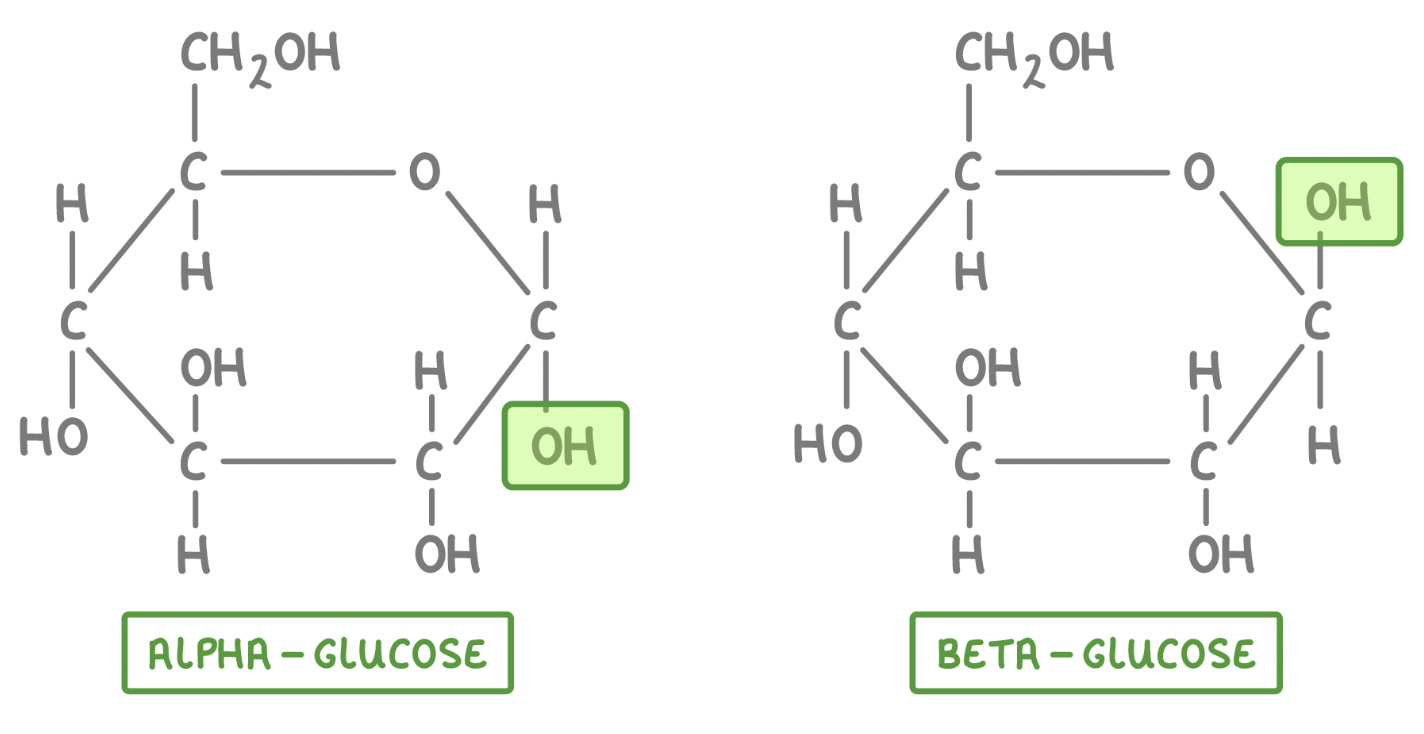
Beta Glucose
the enzyme of beta glucose causes alternating pattern of monomers
Isomeric Fructose Ring
bonding between carbon #1 and #5 or #6 carbon
#6 carbon = alpha-D Fructofuranose
5 sided
#5 carbon = alpha-D Fructopyranose
6 sided
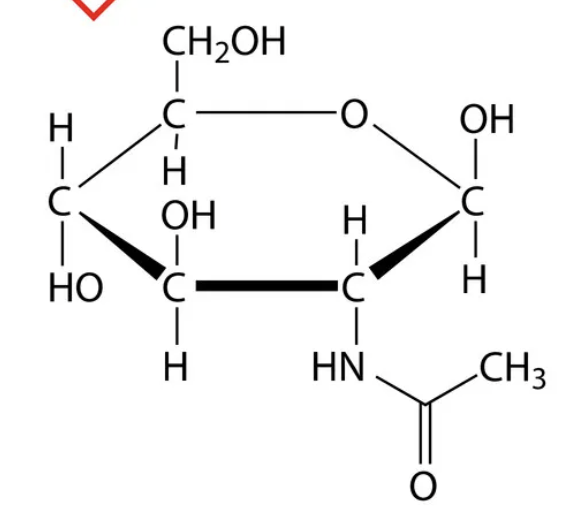
Amino Sugar (NAG)
N-acetylglucoseamine (NAG) is the primary structure of chitin
LIPIDS
fats
phospholipids
steriods
largely insoluble in water due to lack of Nitrogen or Hydrogen (AKA: hydrophobic)
not a true polymer
Fats
Composed of:
1 glycerol backbone
3 fatty acid chains (highly water insoluble)
synthesized by dehydration reactions
Glycerol
not considered a saccharide due to its lack of carbonyl group
considered an alcohol
Fatty Acids
long carbon chains
absolutely PACKED with energy
made 2 carbons at a time
food is broken down 2 carbons at a time and sequestered to form fatty acids (Acetyl CoA) to preserve excess energy
have two ends that help name them
alpha
omega
ALPHA End: fatty acids
the carboxyl group end, in the form of carboxylic acid (COOH)
OMEGA End: fatty acids
the methyl group end (CH3)
Ester Bonds
the linkage between fatty acid’s CARBOXYL group and glycerol’s HYDROXYL group needed for form fats
What are Fats Used For?
energy storage
organ cushioning
insulation
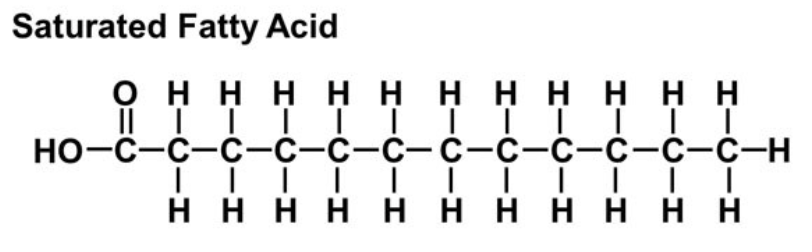
Saturated Fatty Acids
~NO DOUBLE BONDS~
can become solid at room temperature
can pack tightly together
typically made by animals
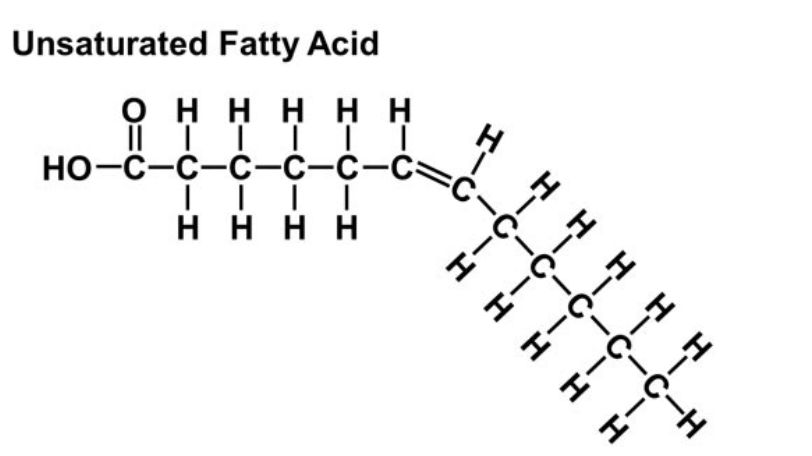
Unsaturated Fatty Acids
~CONTAINS DOUBLE BONDS~
produces a bend in fatty acid chain
typically fluid at room temperature
cannot pack tightly together
contain less hydrogen
typically made by plants
polyunsaturated = 2+ double bonds
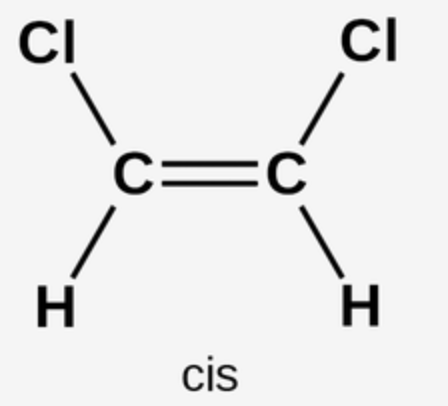
Cis-Double Bonds
breakdown more easily
cause less health risk
vegetable oils
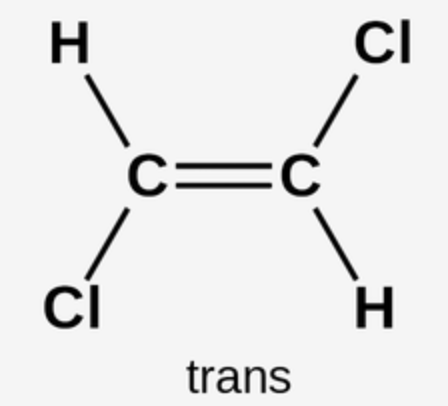
Trans-Double Bonds
stack well in arteries
cause heart disease
a byproduct of hydrogenation
HYDROGENATION
an attempt to turn fragile cis-unsaturated fat into a saturated fat, to increase shelf-life
chemically forcing hydrogen into fat
removing the double bonds
also produces trans-unsaturated fats by accident
ESSENTIAL Fatty Acids
Linoleic Acid → omega-6
Alpha Linolenic Acid → omega-3
free fatty acid
Phospholipids
key component of cellular membranes
phospholipid bilayer
contributes to semipermiability
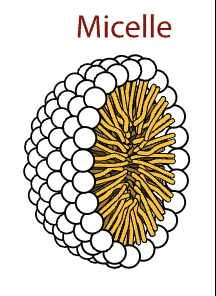
also form little spheres
micelle
liposome (delivery)
Micelle
a sphere
Liposome
hollow sphere
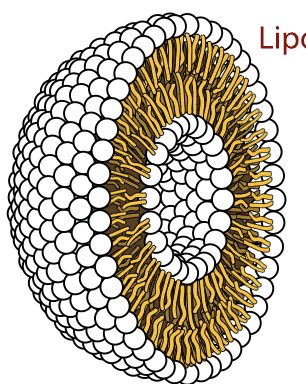
Phospholipid: STRUCTURE
hydrophilic head:
phosphate group (reason for hydrophilic nature)
glycerol
hydrophobic tails:
2 fatty acids
saturated or unsaturated
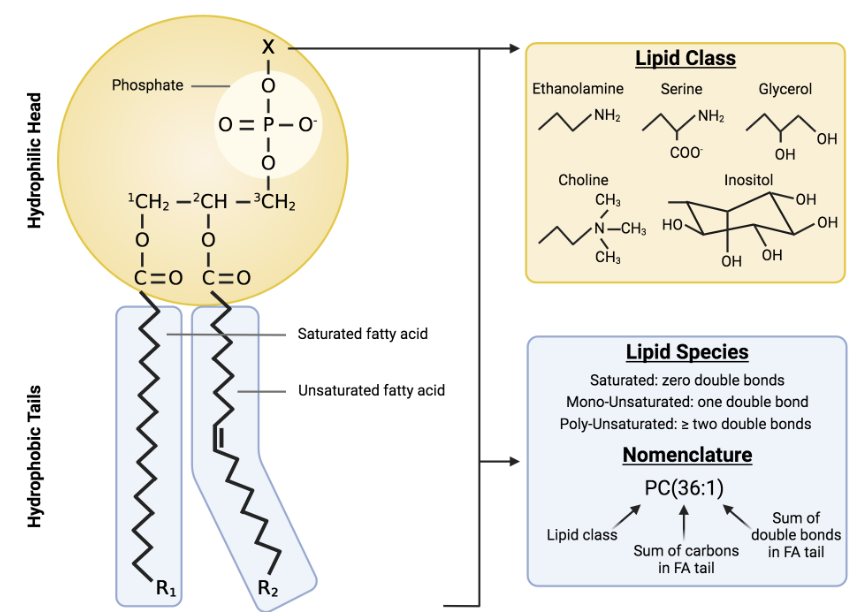
Sphingolipids
a type of phospholipid/membrane molecule
important in certain classes of cellular signalling and the structure of lipid rafts in mammalian systems
composed of → 2 fatty acid chains, glycerol and hydrophilic head
Steriods
a 4 ringed structure with specialized attached side chains
virtually completely hydrophobic/nonpolar
make up most humane hormones
Steroids: CHOLESTEROL
a steroid that helps produce other steroids
7-Dehydrocholesterol is the pre curser to serum cholesterol, converted to vitamin D by ultraviolet light
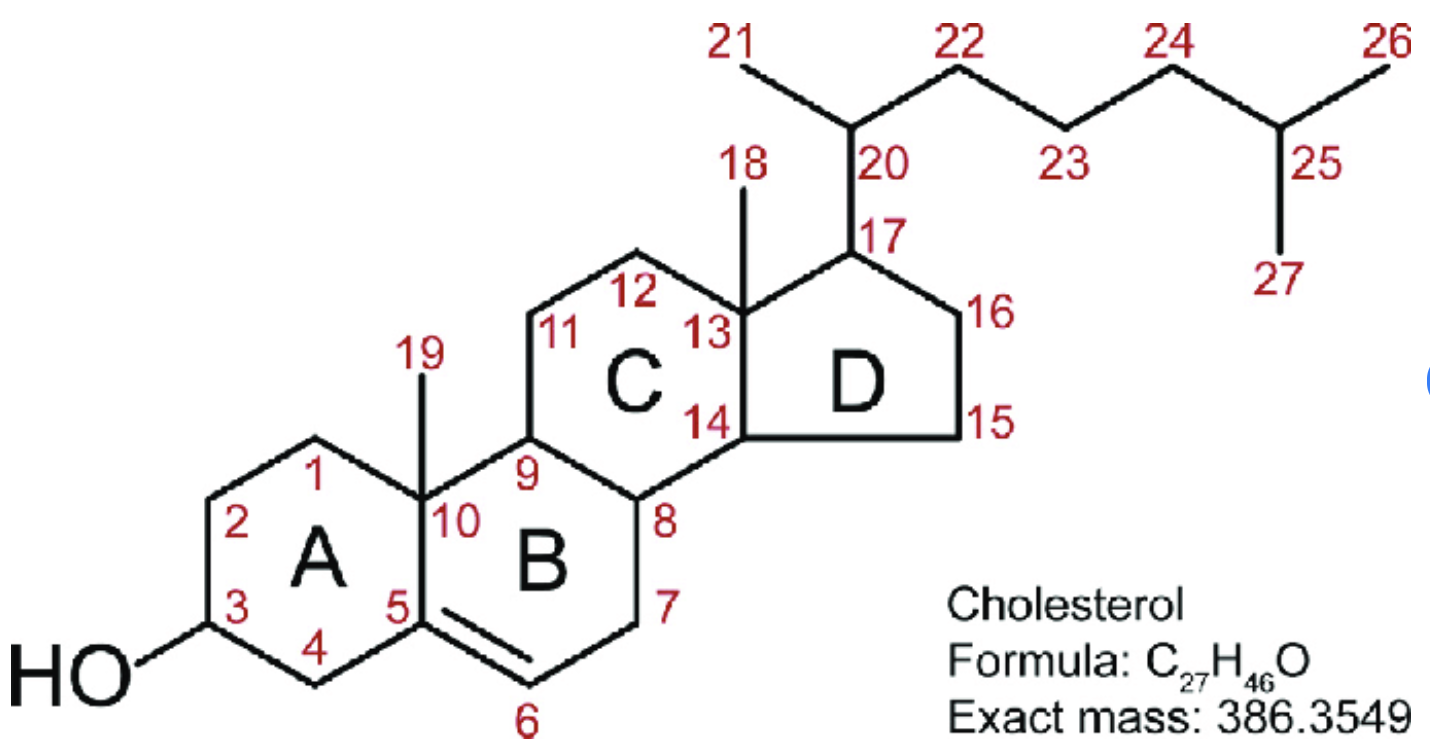
Steroids: NUMBERING
carbon numbering always starts on the top of ring A
PROTEINS
arguably the most important constituent of cells
they do a great variety of jobs within cells
enzymatic
storage
defensive
transport
hormonal
receptor
contractile and motor
structural
ENZYMATIC Proteins
biological catalysts
function → selective acceleration of chemical reactions
bind to specific substrate using Induced Fit
“ase”
example-sucrase
STORAGE Proteins
function → storage of amino acits
example-caesin (milk) is a source of amino acids for baby mammals
DEFENSIVE Proteins
function → protection against disease
example-antibodies deactivate and destroy foreign pathogens
TRANSPORT Proteins
function → transport substances
example-hemoglobin
HORMONAL Proteins
functions → coordination of an organism’s activities
example-insulin, hormone secreted by the pancreas
RECEPTOR Proteins
function → response of cell to chemical stimuli
example-membrane receptor proteins
CONTRACTILE & MOTOR Proteins
function → movement
they change shape in response to a trigger (ATP hydrolysis)
example-motor proteins undulate cilia and flagella
STRUCTURAL Proteins
function → support
example-keratin in hair and horns
Protein vs Polypeptide
Protein - modified polypeptide
Polypeptide - a combination of monomers (amino acids) not yet functional/finished
Protein Polymer
polypeptide
bonded by peptide bonds
Protein Monomers
amino acids
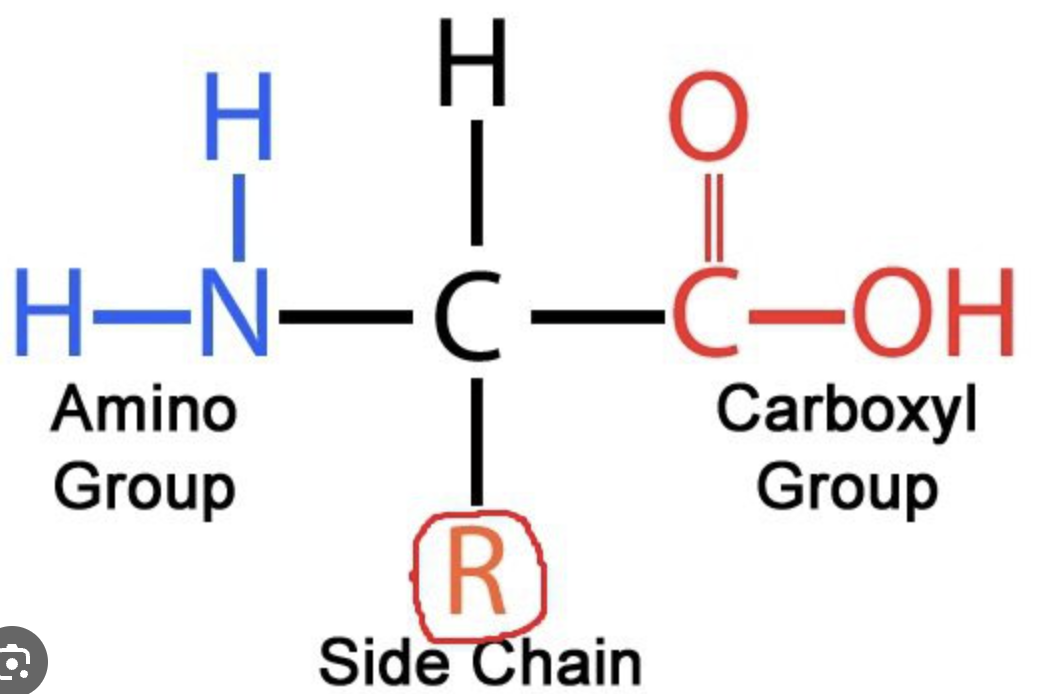
AMINO ACID: Structure
BACKBONE
amino group
central/alpha carbon
carboxyl group
SIDE CHAIN
R group
humans use 20 different amino acids - 11 are synthesized metabolically - 9 are obtained through diet (essential amino acids)
AMINO ACIDS: 20 Human Ones
nonpolar
Glycine (gly)
Alanine (ala)
Valine (val)
Leucine (leu)
Isoleucine (ile)
Methionine (met)
Phenylalanine (phe)
Tryptophan (trp)
Proline (pro)
polar
Serine (ser)
Threonine (thr)
Cysteine (cys)
Tyrosine (tyr)
Asparagine (asn)
Glutamine (gln)
electrically charged
Aspartic Acid (asp)
Glutamic Acid (glu)
Lysine (lys)
Arginine (arg)
Histidine (his)
Types of R Groups/Side Chains
polar (hydrophilic) [6]
generally have a hydroxyl group (OH)
non-polar (hydrophobic) [9]
generally contain only carbon and hydrogen
electrically charged (hydrophilic) [5]
acidic/carboxyl end
negatively charged side chain
basic/amino end
positively charged side chain
determine the type of amino acid and has a great deal of affect on protein shape and structure
Enzymes
biological catalysts (usually proteins)
act on SUBSTRATE specific to the ACTIVE SITE of the enzyme
Zwitterion
amino acids ionized via the donation of OH’s hydrogen to the amino group
done inside cytosol
LEVELS of PROTEIN STRUCTURE
primary
secondary
tertiary
quaternary
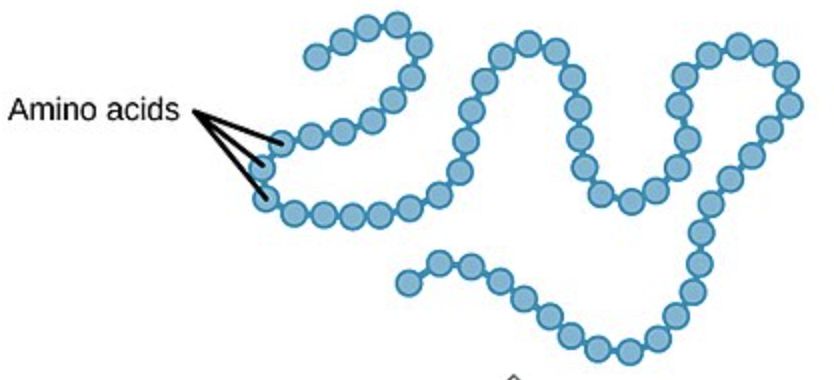
PRIMARY Protein Structure
linear chain of amino acids in SPECIFIC SEQUENCE
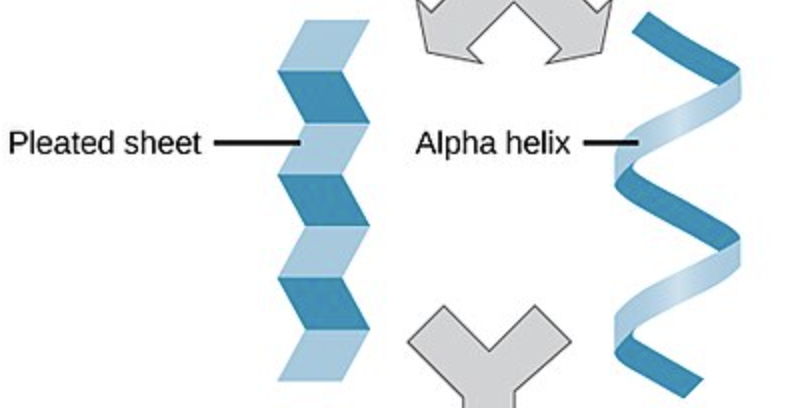
SECONDARY Protein Structure
hydrogen bonding between backbones of amino acids determining primary function
alpha helix
beta pleats
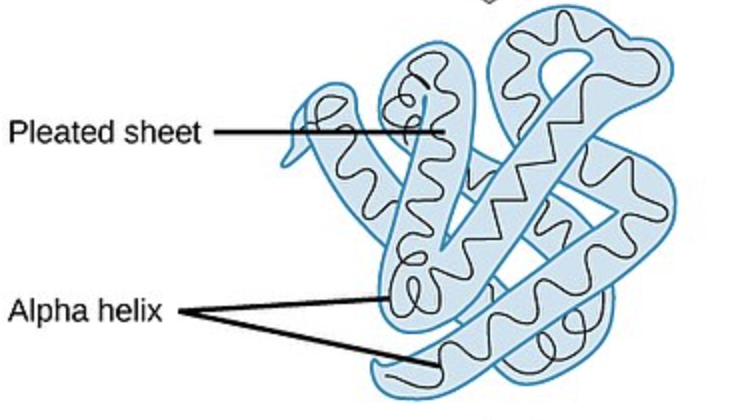
TERTIARY Protein Structure
side chain interactions via:
hydrogen bonds (weak)
disulfide bridges (strong)
hydrophobic interactions
van der Waals interactions
ionic bonds (weak)
forming a 3D structure
QUANTERNARY Protein Structure
the combination of multiple tertiary structure proteins via:
hydrogen bonds
ionic bonds
disulfide bridges
hydrophobic interactions
2 or more tertiary structure polypeptides
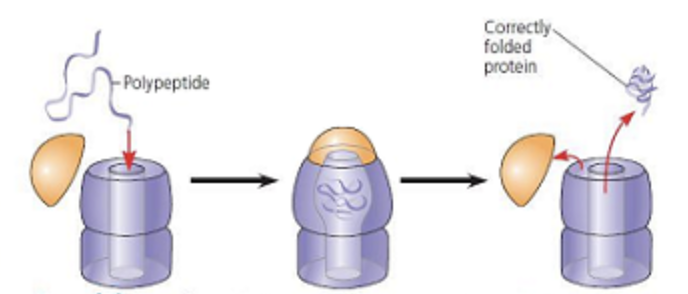
Chaperonin
a structure that functions as a space for polypeptides to fold properly into proteins
3 part protein and nucleic acid based structure
when cap attaches a hydrophobic environment is formed inside the “pocket space”
this stimulates proper form
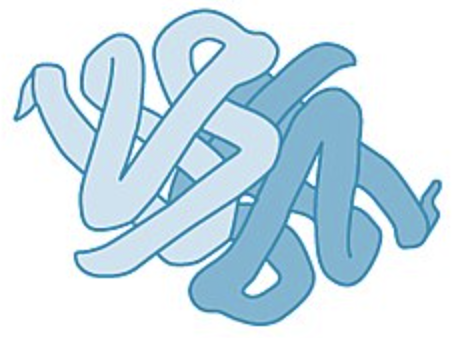
Native Form
the naturally occurring protein prior to denaturation
Denaturation
the breaking-down of protein via unfolding and bond breaking
deactivates protein
can be caused by pH, temperature, and ion concentration changes
Renaturation
the reformation of protein via refolding and bond reattaching
only possible to reform weak interactions
reactivates protein
NUCLEIC ACIDS
store and transmit hereditary information
primarily encode information for proteins
DNA and RNA
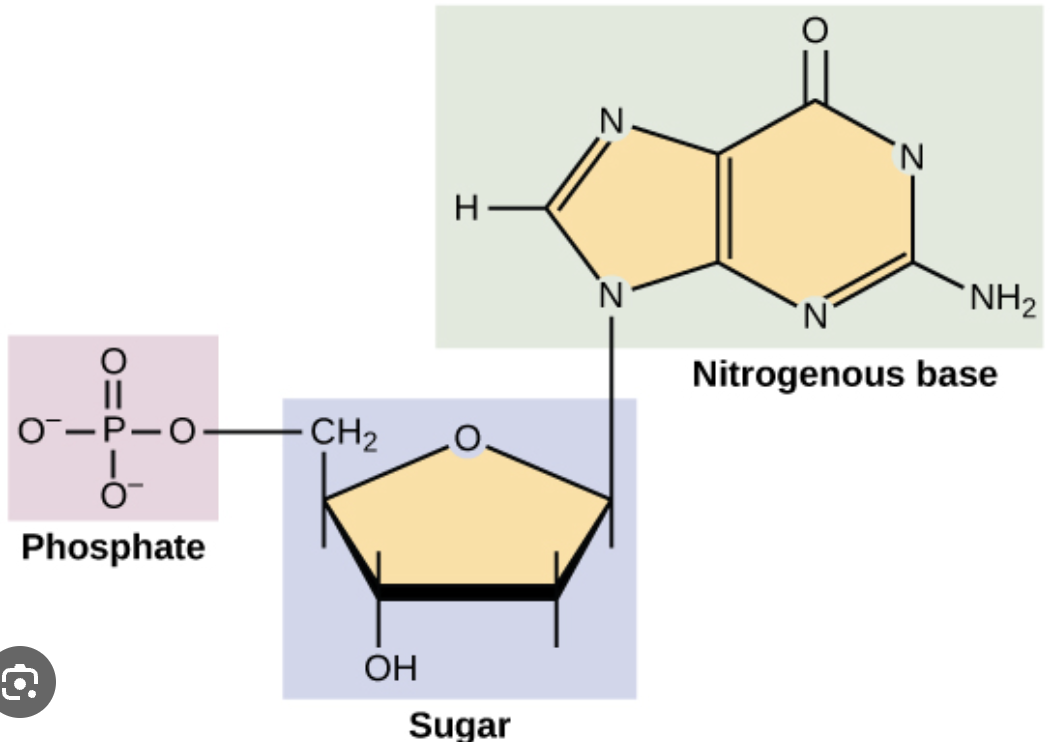
Nucleic Acid Monomer
NUCLEOTIDES
many nucleotides bonded by phosphodiester bonds produces polynucleotides (polymer)
examples - DNA and RNA nucleotides
Phosphodiester Bonds
covalent bond between nucleotide 1’s #3 carbon and nucleotide 2’s #5 carbon of each’s pentose sugar
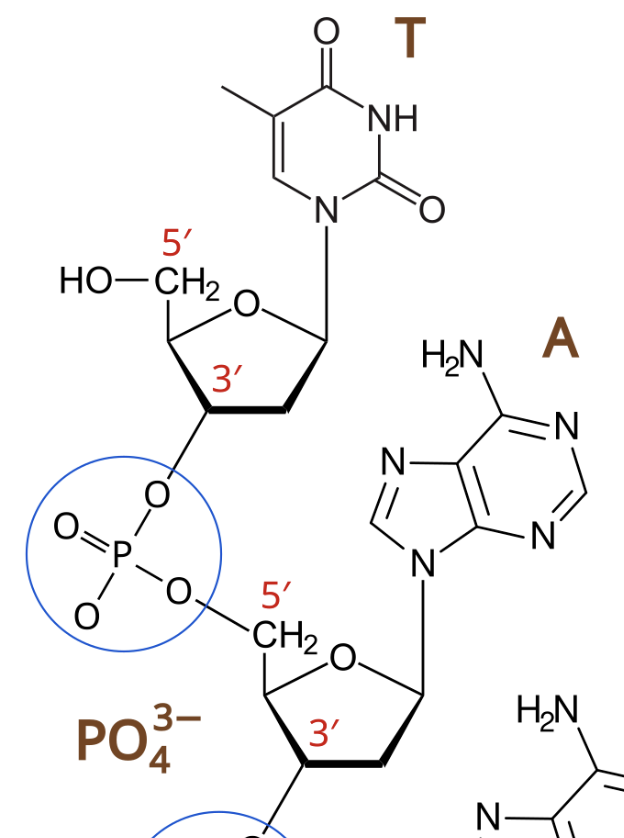
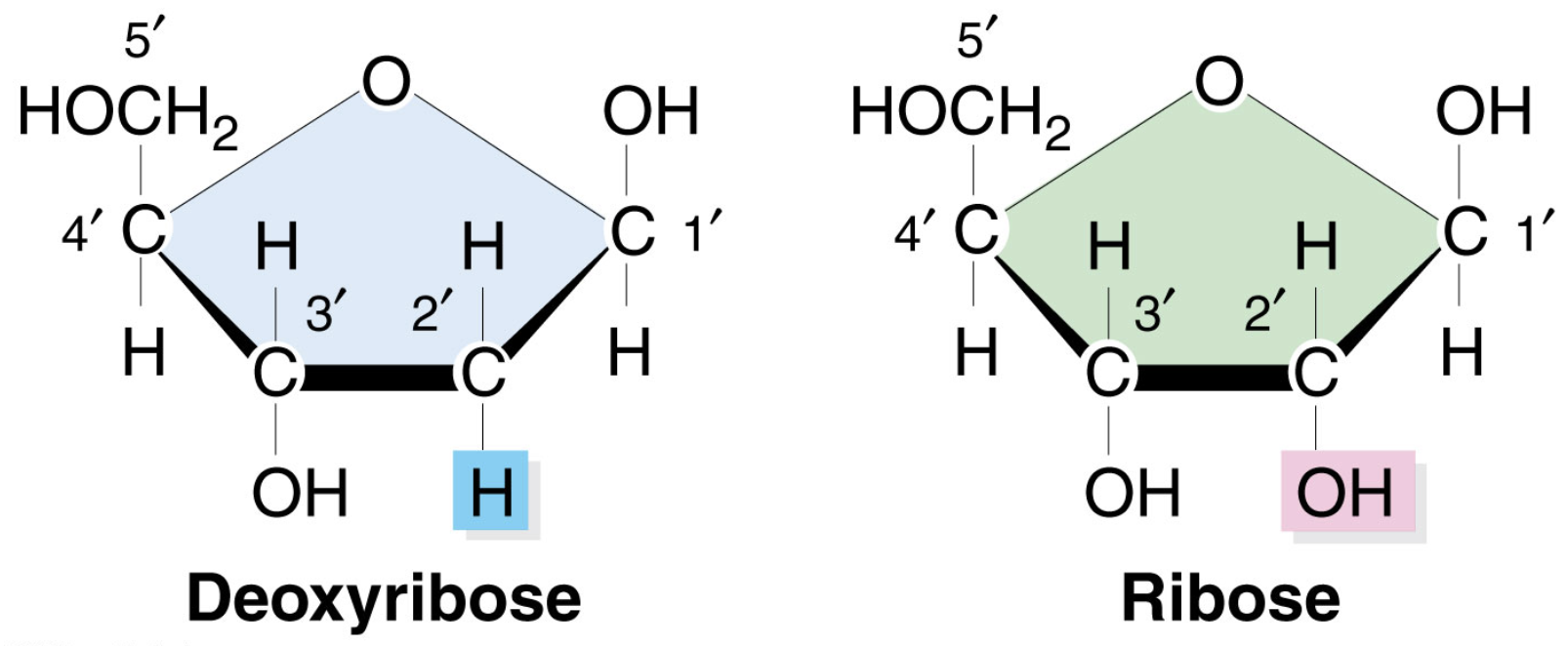
Types of Pentose Sugar
Ribose (OH)
Deoxyribose (H)
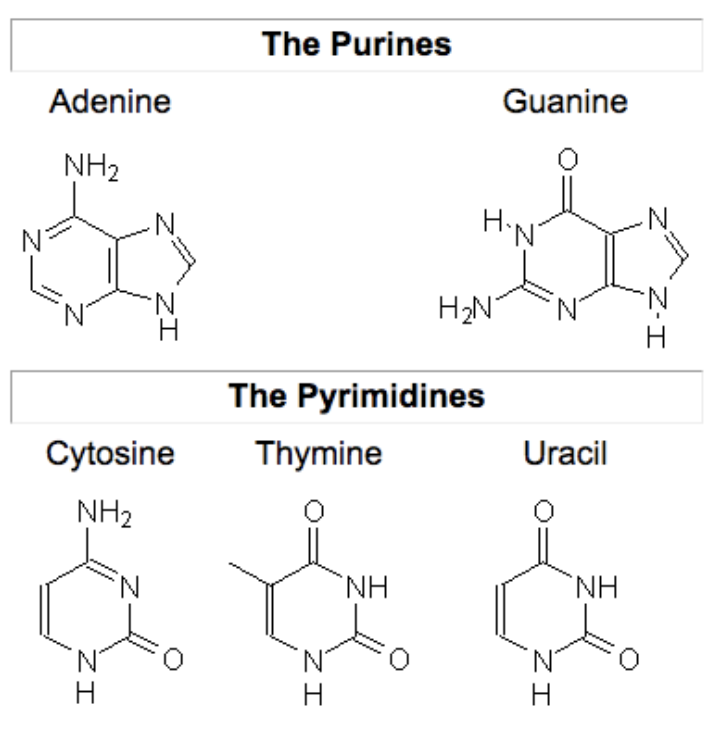
Types of Nitrogenous Bases
Pyrimidines (one ring)
Cytosine (C)
Thymine (T)
Uracil (U)
Purines (two rings)
Adenine (A)
Guanine (G)
Sugar-Phosphate Backbone
a series of nucleotides bonded by phosphodiester bonds
5’ prime to 3’ prime
DNA Macromolecules: Structure
double stranded
uses deoxyribonucleotides
genetic material
does not use Uracil
DOUBLE HELIX
Sugar-Phosphate Backbone
Nitrogenous Bases
polynucleotides strands prefer double-strand configuration
DOUBLE HELIX
two strands of bonded nucleotides hydrogen bonded by nitrogenous bases
bases must be properly paired and orientated
strands are complementary antiparallel
two lane highway
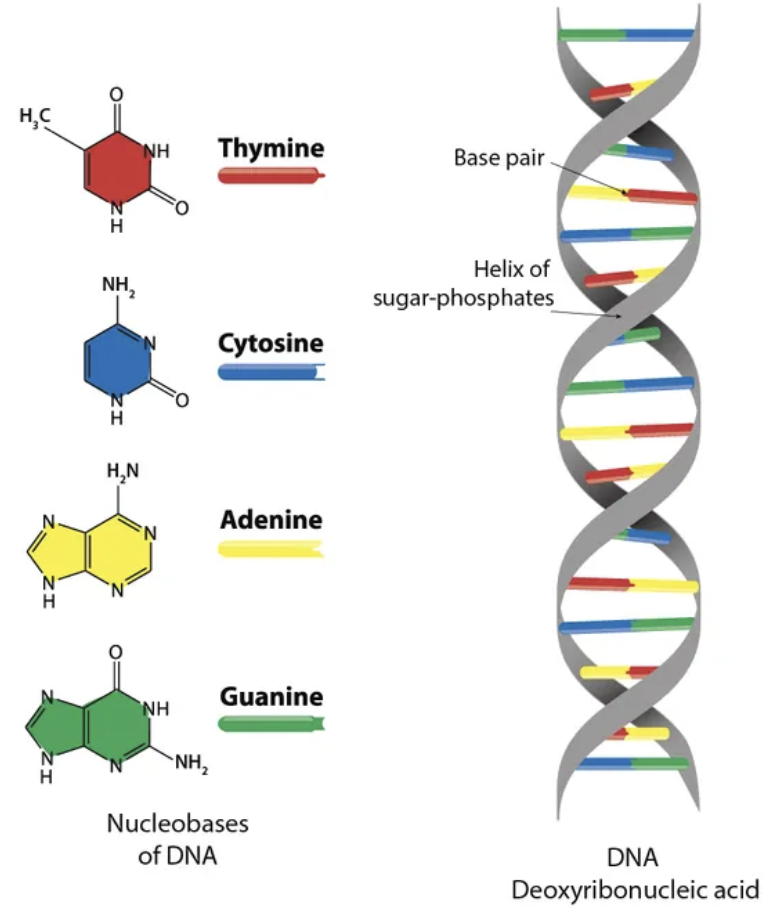
Base Pairs
Adenine - Thymine (2 hydrogen bonds)
Guanine - Cytosine (3 hydrogen bonds)
RNA Macromolecule: Structure
single strand
uses ribonucleotides
not genetic material but transport for it
uses Uracil not Thymine
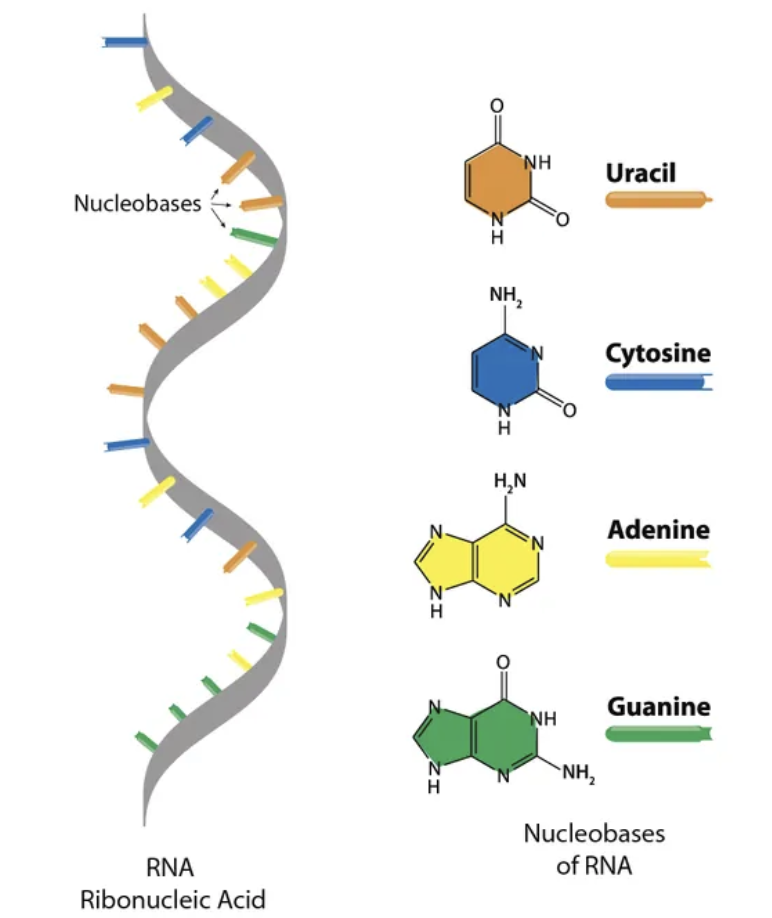
RNA Classifications
mRNA
messenger
rRNA
ribosomal
tRNA
transfer
bring amino acids to ribosome
small RNA
small nuclear
splicsosomes
CELLS
the functional unit of life
organisms can be multicellular or unicellular
Cell Theory States
all living things are comprised of cells
Robert Hooke
developed one of the first microscope that used reflective light
Anton Van Leeuwnehoek
developed light transmitted microscope
Electron Microscopes
uses electron beams, with very small wavelengths increasing resolution, rather than light
Two types:
Scanning Electron Microscope
surface view of specimen '“SEM”
Transmission Electron Microscope
internal view of sample “TEM”
cut slices then view
Prokaryotes
simple cells
no membrane bound organelles
no proper chromosomes
found in nucleoid region
Plasma Membrane (external)
coated in Cell Wall infused with Peptidoglycan
protein/carbohydrate based Capsule may coat the cell
this is a sticky shell that helps bond colonies and slow down WBC
Fimbriae (hair like) help with bonding and adherence
Flagella helps with motility
one or more
structure is different in eukaryotes
No Histones
Eukaryotes
more complex
many membrane bound internal structures
significantly higher surface area
ENDOMEMBRANE SYSTEM to combat this
two types
Animal vs Plant Cells
Animal
no cell wall
no central vacuole
no chloroplasts
centrosome
lysosome
Plant
cell wall
central vacuole
chloroplasts
not regular centrosome
no lysosomes
ENDOMEMBRANE SYSTEM
membrane surfaces are run through the cell to bring SA:V ratio to better range
functions to transport proteins through and out of the cell, also modifies peptides
extra membrane gives space for biological processes (most of which occur along membrane)
basically any membrane based organelles except mitochondrion, chloroplasts, peroxisomes
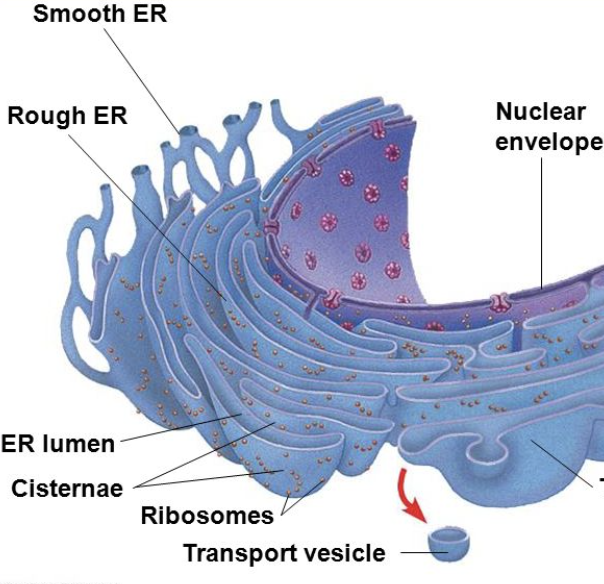
Nuclear Envelope: endomembrane system
double membrane
inner and outer
flows seamlessly into Rough ER
encloses around Chromatin and Nucleus
has pours for extra-envelope transfer
Nucleolus
not a membrane bound section but an organized portion of nucleus
this is where ribosomes are born (put together)
Nucleosomes
“beads” made of several histone proteins wrapped tightly with DNA strands. many nucleosomes result in chromatin
~10nm diameter
Nuclear Lamina: endomembrane system
fibrous matrix
located against inner nuclear envelope membrane
provides envelope extra support and defense
Ribosomes: endomembrane system
function: synthesis of proteins, by translating mRNA and assembly of coded amino acid chains
made of ribosomal RNA and ribosomal proteins
Two subunits
Large Subunit
Small Subunit
subunits remain separate until active transcription/translation
can be free floating or bound to ER
Rough Endoplasmic Reticulum (ER): endomembrane system
has ribosomes bound to it
function:
primarily to produce secretory proteins (modified)
outside the cell or to organelles
site of phospholipid formation
this synthesizes more endomembrane
post-translational modifications of peptides
attached to nuclear envelope
protein assembly line
Smooth Endoplasmic Reticulum (ER): endomembrane system
no ribosomes
function:
synthesis of lipids
steroids and phospholipids
detoxifies poisons and drugs
site of glycogen metabolism
storage of calcium
sarcoplasmic reticulum
also some post-transcriptional protein modifications
vesicles bud off here
export
Cisternae: endomembrane system
the folds of the ER
has its own Lumen → the inner area/pockets formed by folds
begins modifications
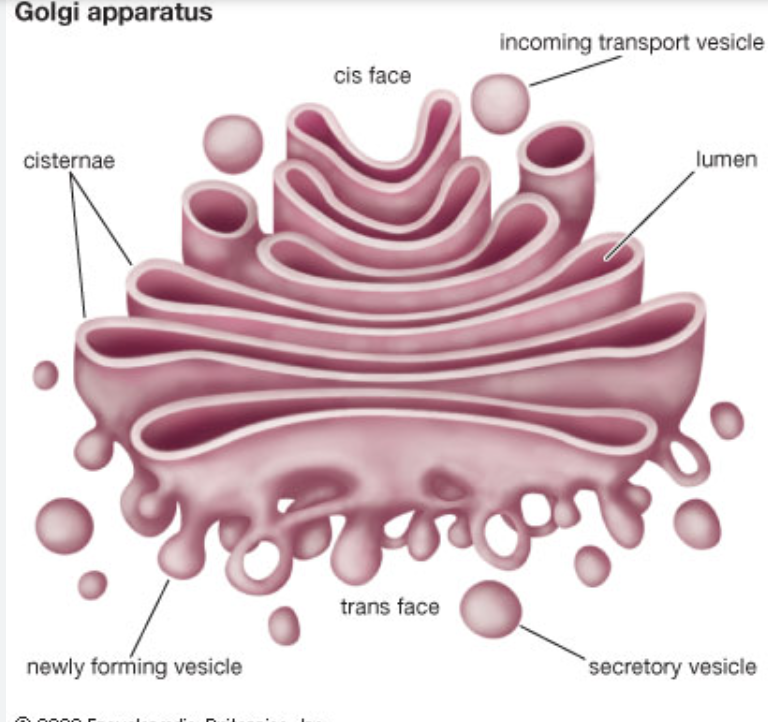
Golgi Apparatus: endomembrane system
a series of “flattened sacks”
function:
further modification of proteins for exocytosis
proteins are received via vesicles on the CIS FACE
protein is taken up via golgi cisternae and modified
packaged into vesicle and shipped out via TRANS FACE
motor proteins help vesicles move between “sacks”
Common Protein Modifications
chain cleaving/shortening
attachment of
carbs
lipids
other proteins
folding and shape changes