Free Radical Polymerization
1/32
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
33 Terms
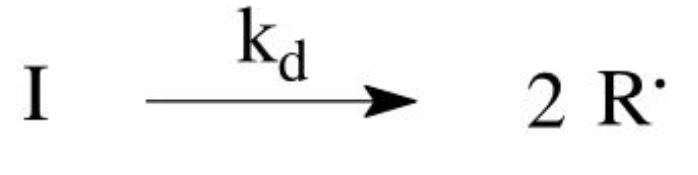
Decomposition mechanism
Addition mechanism
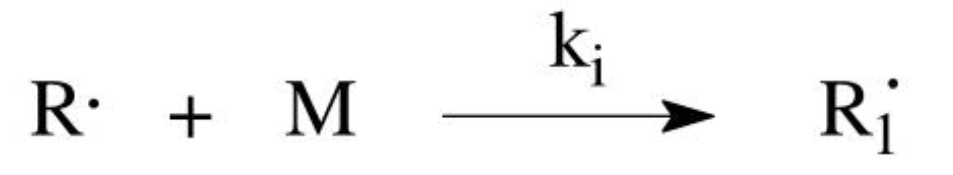
Radical production rate
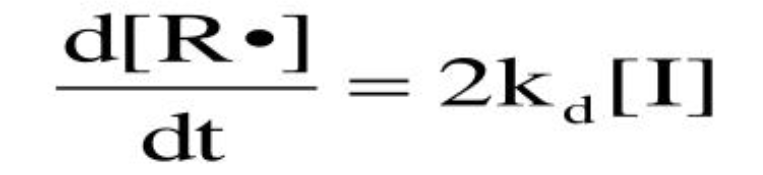
Propagation mechanism
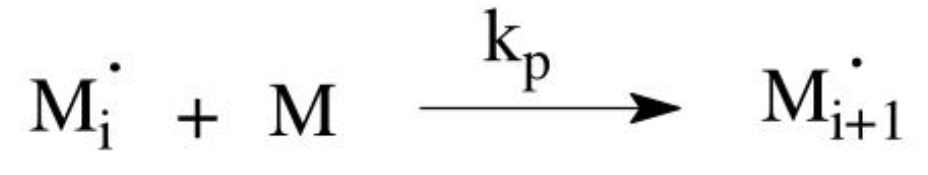
Propagation rate
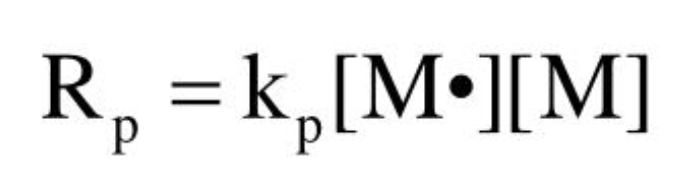
Initiator efficiency
Takes into account the fact that only some of the radicals from the initiator actually react with monomers to initiate growing chains
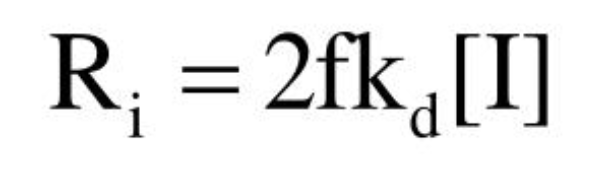
Mutual termination
two growing polymer chains react with each other to stop the polymerization process. Rarely occurs due to low concentration of growing polymer chains
Combination
the radicals collide and form a new covalent bond
Combination mechanism
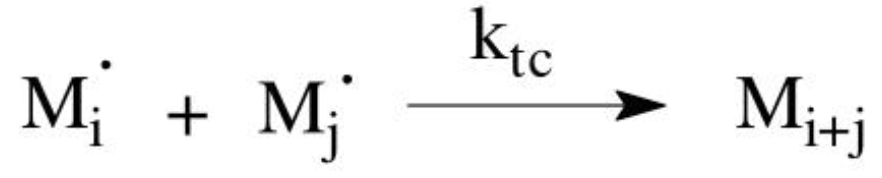
Disproportionation
involves the transfer of a proton from one chain to another
Disproportionation mechanism
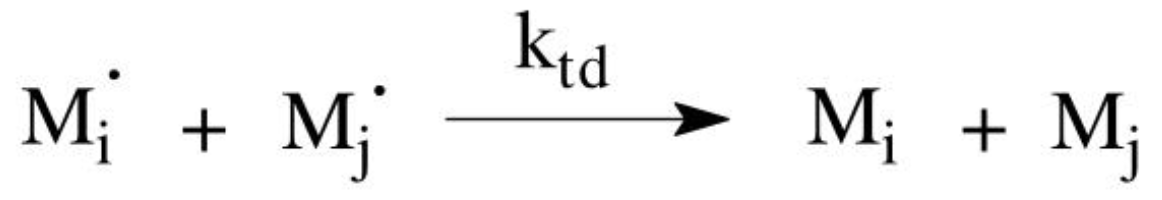
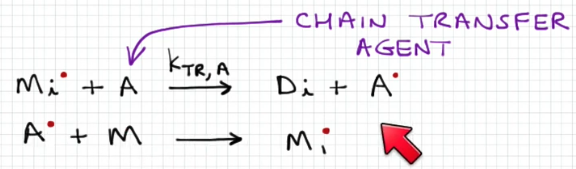
Chain transfer
An atom is transferred to the growing chain, terminating the chain and starting a new one
Chain transfer mechanism
General termination rate
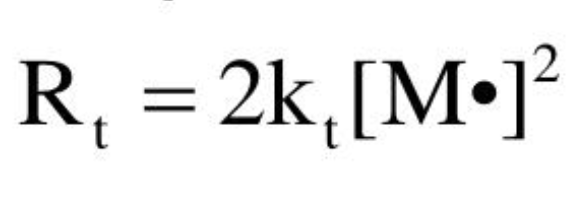
Rate of polymerization
rate at which monomer is consumed
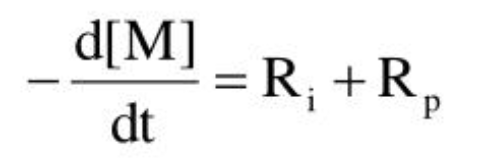
B/c Rp»Ri
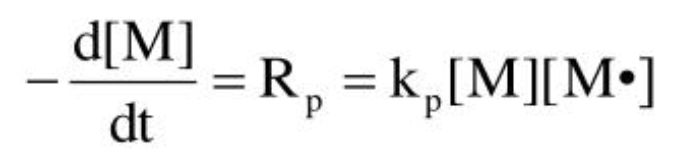
Quasi-steady state assumption
the concentration of free radicals remains constant (consumed at same rate as generation)
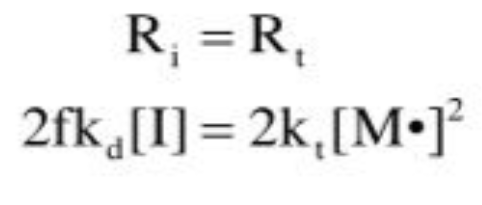
SS monomer concentration
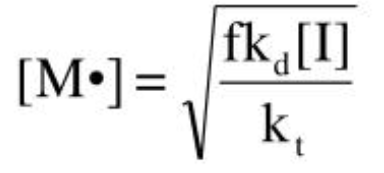
SS rate law
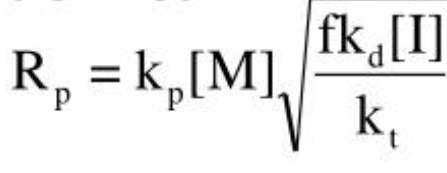
kinetic chain length equation
average number of monomers polymerized per chain radical
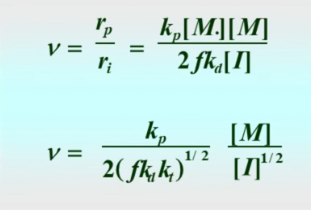
Kinetic chain length
equals DP for disproportionation and is twice DP for combination
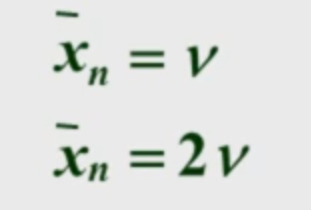
Carothers theory
Assumes that the intrinsic reactivity of a functional group is independent of molecular size and is not impacted by the reaction of the other group
Increases polymerization rates
Temperature
Monomer/initator concentration
Solvents
Summary of mechanisms
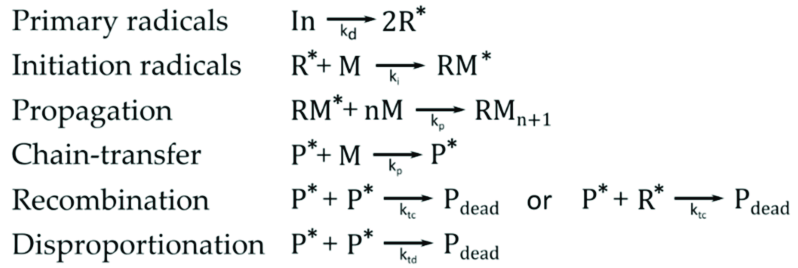
Copolymerization
Mechanisms and reactivity ratios of
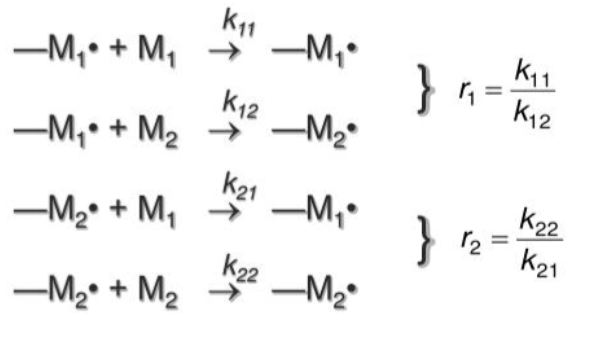
Impact monomer reactivity
steric hindrance
resonance stability at the radical site
double bond polarity
r1=0 and r2=0
Each comonmer prefers to react with each other resulting in alternating copolymer
r1>1 and r2>1
Each comonomer prefers to react with others of its own kind resulting in block copolymers
r1*r2=1
No preference due to chain ends resulting in random copolymer
r1>1
homopolymerization growth is preferred
r1=0
only reaction with monomer 2 will occur
Composition of monomer
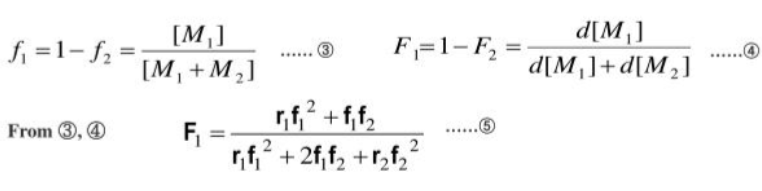
Ideal copolymerization
