Reactions of Alkenes
1/14
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
15 Terms
1) H2, Pd/C or Pt
hydrogenation, forms alkane
syn addition
1) Hg(OAc)2, H2O or ROH
2) NaBH4
alkoxymercuration, forms OH
markovnikov
OH from H2O adds to area with less H
H adds to area with more H
1) BH3•THF 2) H2O2, OH–
hydroboration, forms OH
anti-mark
H adds to area with LESS H
OH adds to area with MORE H
syn-additon
1) X2, H2O (e.g., Br2, H2O)
halohydrin formation
anti-addition
Cl adds to more H side
OH adds to less H side
cuz OH>Cl
1) mCPBA (RCO3H)
epoxide formation
syn-additon
1) mcbpa/peroxyacid in water
anti-addition
forms two trans OH
epoxide opens in water
1) HX
alkyl halide
mark
Cl to less H side
H to more H side
1) HX, ROOR
alkyl halide
anti-mark
Cl to more H
H to less H
1) X2 (e.g., Br2 or Cl2), CCl4
anti-addition
forms dihalide
1) OsO4, H2O2 or KMnO4 (cold, dilute)
syn addition
OH’s on same side
1) O3
2) DMS or Zn/H2O
or
1) KMO4
ozonolysis, forms aldehyde and ketone
1) KMnO4
2) H3O+
forms a ketone and carboxylate/carbon dioxide (+water), ozonolysis
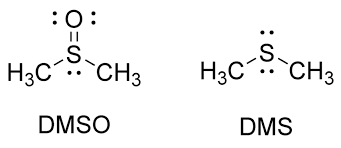
DMSO vs DMS
dimethyl sulfoxide
dimethyl sulfide
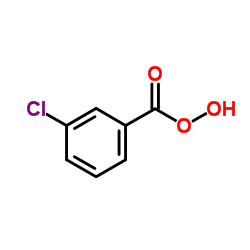
mCPBA
m-Chloroperoxybenzoic acid
1) H2O
2) H3O+/H+
hydration, forms OH
mark