orgo t1-3 naming
1/47
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
48 Terms
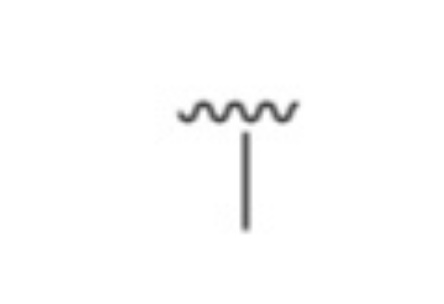
what is it?
Methyl
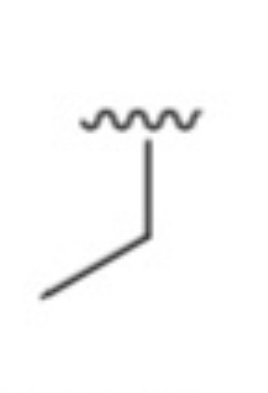
What is it?
Ethyl
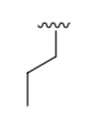
What is it?
Propyl
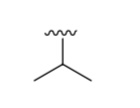
What is it?
Isopropyl
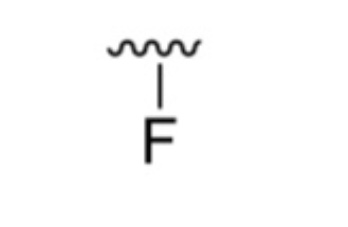
What is it?
Fluoro
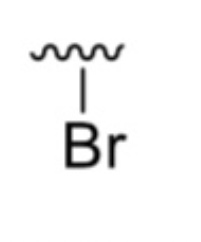
What is it?
Bromo
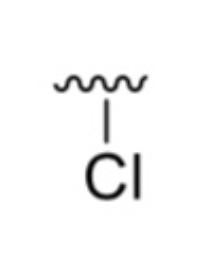
What is it?
Chloro
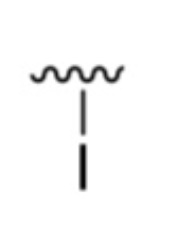
What is it?
Iodo
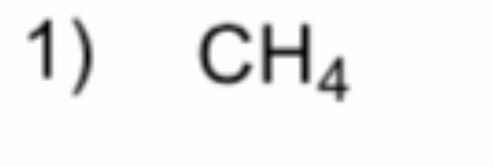
What is it?
Methane
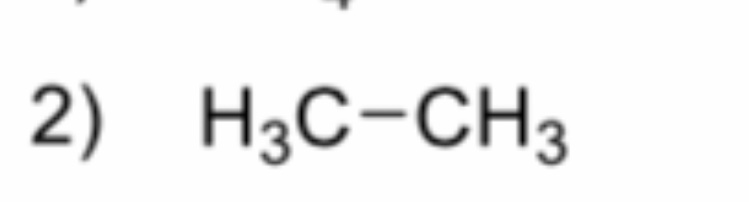
What is it?
Ethane
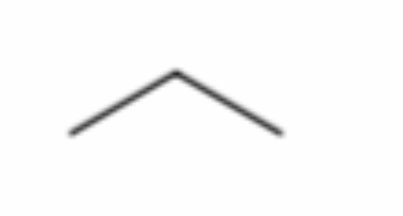
What is it?
Propane
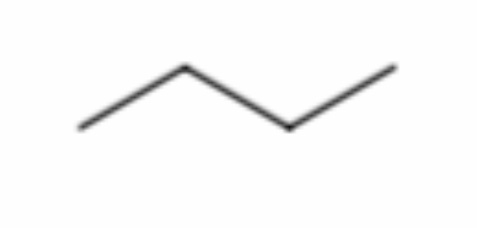
What is it?
Butane
5 carbons
Pentane
6 carbons
Hexane
7 carbons
Heptane
8 carbons
Octane
9 carbons
Nonane
10 carbons
Decane
Prefix 2
Di
Prefix 3
Tri
Prefix 4
Tetra
Prefix 5
Penta
Prefix 6
Hexa
Prefix 8
Octa
Prefix 9
Nona
nonpolar
cancel or go opposite (equal sharing). no lean towards one specific thing it is equal
polar
molecules go/lean towards one main molecule
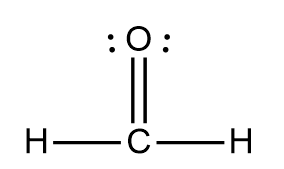
H2CO - Bonds: carbon 4 and O wants 8
largest last group/valence (2s², 2p^4). Count the exponent or arrows O=6 dots/ form pairs
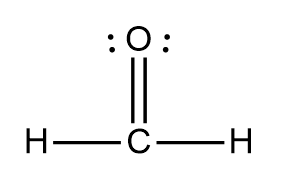
sigma and pi bonds
sigma 3 pi 1
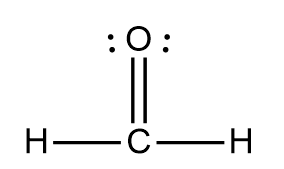
what is the hybridization
sp²
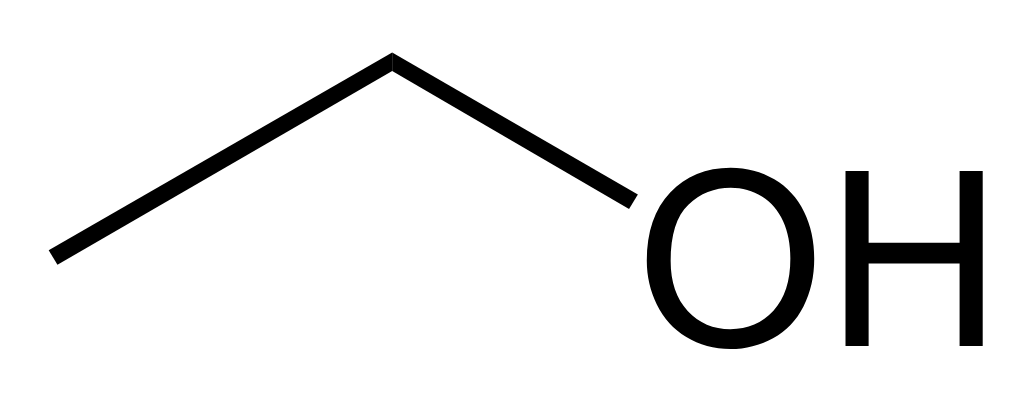
what is it/it can be longer
alcohol
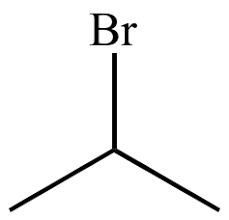
this, but rotated right so that br is on the end/edge
alkyhalides
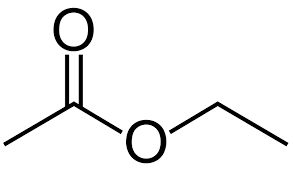
\/\O/ it looks closer this, but it’s the same thing
ester
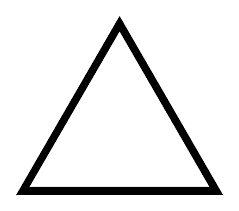
what is it?
cyclopropane
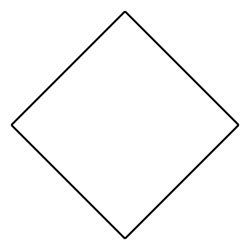
what is it when it is a substituent/hanging off the main chain
cyclobutyl
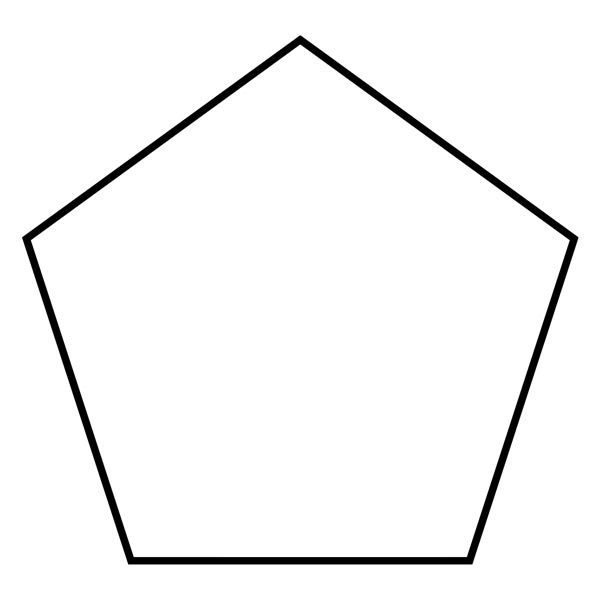
what is it when it is a substituent/hanging off the main chain
cyclopentyl
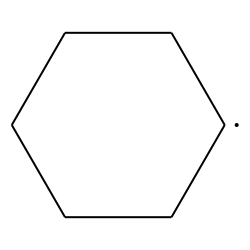
what is it when it is a substituent/hanging off the main chain
cyclohexyl
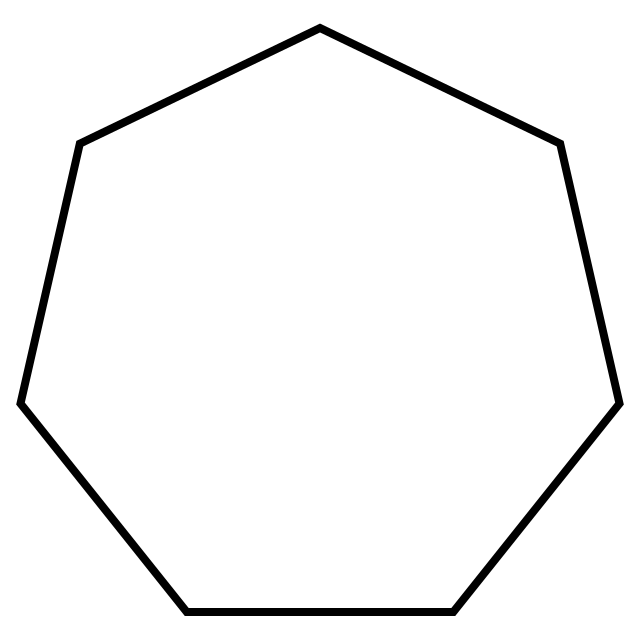
what is it when it is a substituent/hanging off the main chain
cycloheptyl
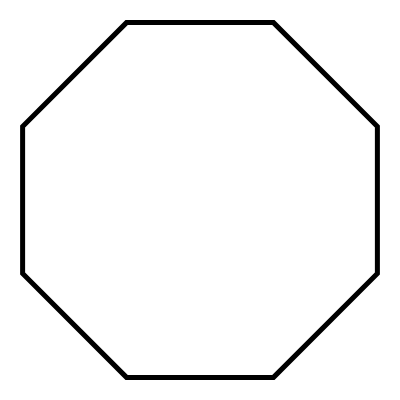
what is it when it is a substituent/hanging off the main chain
cyclooctyl
smaller shapes are…stable
less stable
…are more stable than rings
chains
equitorial
angled and more flexible
axial
straight and limited/no movement
when flipping to a chair: a → and vise versa, but up or down/arrow stays the same direction just change type
e
when flipping to chairs: the a and e might switch, but … does not
direction/up or down
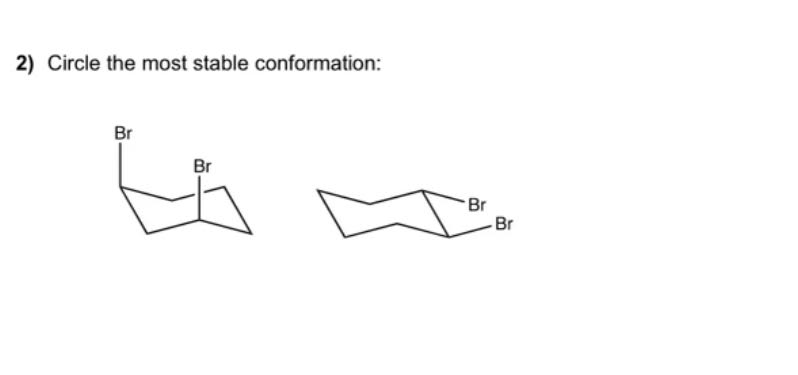
which is the most stable
the right one
only for alkenes (only 1 bond over the line) E=
different directions
only for alkenes (only 1 bond over the line) Z=
same directions