Density of materials
0.0(0)
Card Sorting
1/3
There's no tags or description
Looks like no tags are added yet.
Last updated 3:16 PM on 6/16/23
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
4 Terms
1
New cards
density = mass/volume
Equation for density
2
New cards
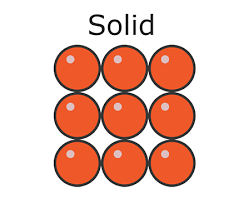
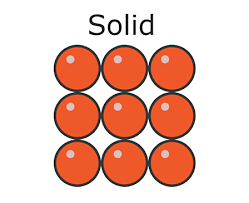
* In a solid:
* The particles are **closely packed**
* The particles **vibrate** about fixed positions
\
* Solids have:
* A definite **shape** (they are **rigid**)
* A definite **volume**
* The particles are **closely packed**
* The particles **vibrate** about fixed positions
\
* Solids have:
* A definite **shape** (they are **rigid**)
* A definite **volume**
Density in a solid
3
New cards
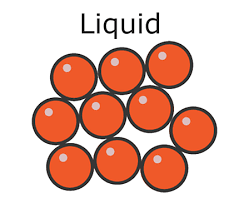
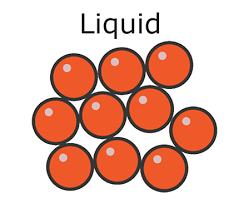
* In a liquid:
* The particles are **closely packed**
* The particles can **flow** over one another
\
* Liquids have:
* No definite shape – they are able to **flow** and will take the shape of a container
* A definite **volume**
* The particles are **closely packed**
* The particles can **flow** over one another
\
* Liquids have:
* No definite shape – they are able to **flow** and will take the shape of a container
* A definite **volume**
Density in a liquid
4
New cards
\
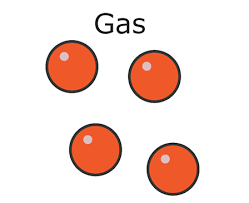
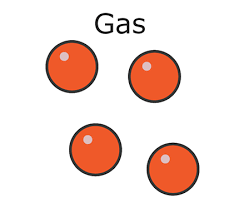
* In a gas:
* The particles are **far apart**
* The particles move **randomly**
\
* Gases have:
* No definite shape
* No fixed volume
* \
* Gases are highly **compressible**, this is because:
* There are large **gaps** between the particles
* It is easier to **push** the particles closer together than in solids or liquids
\
\
\
* In a gas:
* The particles are **far apart**
* The particles move **randomly**
\
* Gases have:
* No definite shape
* No fixed volume
* \
* Gases are highly **compressible**, this is because:
* There are large **gaps** between the particles
* It is easier to **push** the particles closer together than in solids or liquids
\
\
\
Density in a gas