Chemistry (P2) - Functional Group Analysis, Reactions and Mechanisms
1/66
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
67 Terms
State the three main reactions that alkanes undergo
Combustion, cracking and halogenation
Describe alkane combustion
Alkanes undergo combustion in excess oxygen to form carbon dioxide and water and undergo incomplete combustion in a lack of oxygen to form carbon monoxide and water
Describe thermal cracking
The C-C bond of an alkane breaks to give two fragments in which one electron of the bond pair is located on each fragment producing alkyl radicals which lose a hydrogen atom and rearrange to produce hydrocarbons of smaller molecular weights than the original alkane
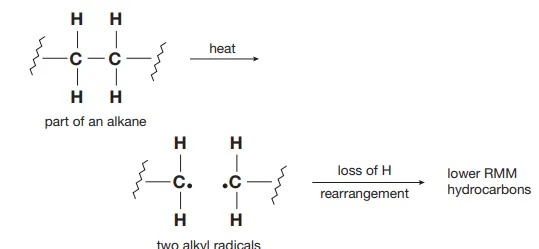
Identify this image
Thermal cracking
Describe catalytic cracking
A zeolite produces positively charged intermediates of the alkane in which it loses a hydrogen atom, forming a alkyl radical which rearranges to form hydrocarbons of smaller molecular weight than the original alkane
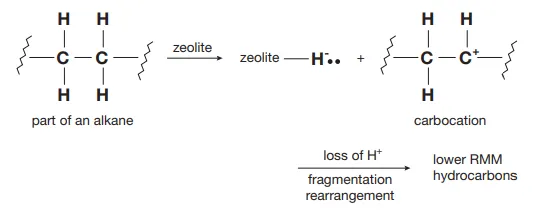
Identify this image
Catalytic cracking
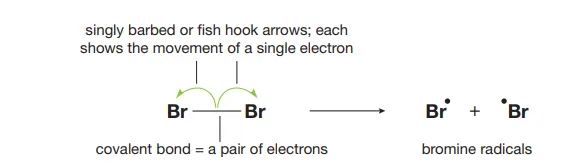
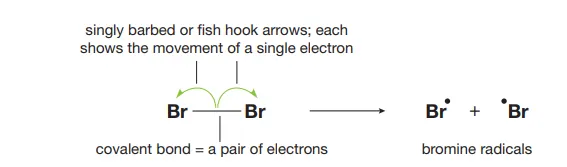
Describe the homolysis of halogens
UV light facilitates the breaking of the covalent bond and each atom retains a single electron from the bonding pair
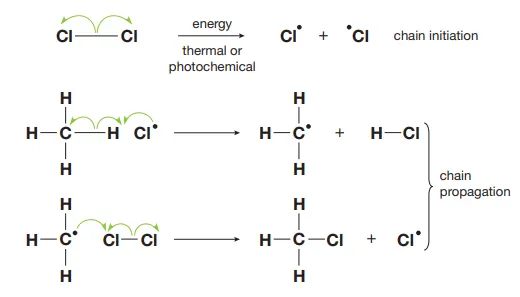
Describe how propagation causes chain reactions and lead to halogenation
Propagation results in a chain of reactions as each propagation creates a new radical which can react with another stable molecule. In the event that an alkyl radical reacts with a halogen molecule, halogenation can occur.
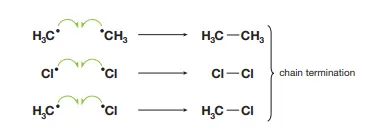
Describe how termination slows down chain reactions and lead to halogenation
The more radicals that react, the less radicals there are to continue the propagation. Halogenation can occur when a halogen radical reacts with an alkyl radical
Describe the reactions between primary and tertiary halogenoalkanes and hydroxide ions respectively
They react to form primary and tertiary alcohols respectively
Identify this diagram
Initiation
Identify this diagram
Propagation
Identify this diagram
Termination
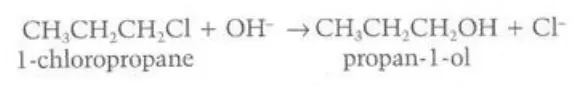
Identify this diagram
Primary halogenoalkane reacting with hydroxide ion to form primary alcohol
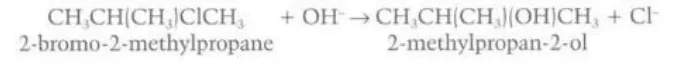
Identify this diagram
Tertiary halogenoalkane reacting with hydroxide ion to form tertiary alcohol
Describe the mechanisms behind the conversion of primary/tertiary halogenoalkanes into alcohols
The bond between the halogen and the carbon creates a region of electron-deficiency around the carbon atom. The hydroxide ion acts as a nucleophile and donates a pair of electrons to the carbon, forming a bond between itself and the carbon atom and breaking its bond with the halogen, allowing the halogen to gain an electron from the carbon and become a negatively charged free radical
How can we identify the halogen group in a halogenoalkane? Describe the test
Each halogenoalkane produces a different halide precipitate. The test involves hydrolysing the halogenoalkane with sodium hydroxide and adding excess nitric acid to neutralize the hydroxide ions. After which, a few drops of aqueous silver nitrate are added which results in the precipitation.
Chloroalkanes give a white precipitate which darkens rapidly. Bromoalkanes give a cream precipitate which darkens slowly. Iodoalkanes give a light yellow precipitate which does not darken
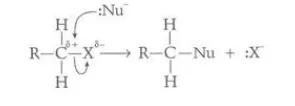
Identify this image
Nucleophilic substitution via homolysis
State the three main reactions which alkenes undergo
additions with bromine, reactions with potassium manganate (VII) and additions with hydrogen halides
Describe the reaction between liquid bromine and alkenes
Diatomic bromine acts as an electrophile and the high electron density in the C=C bond repels the electron pair in the molecule which polarizes bromine and the positive dipole attacks the area of high electron density and forms a bond with one of the carbon atoms, creating a positive carbocation which is then attacked by the other bromide ion created when the other bromine atom gained the electron pair from the molecule
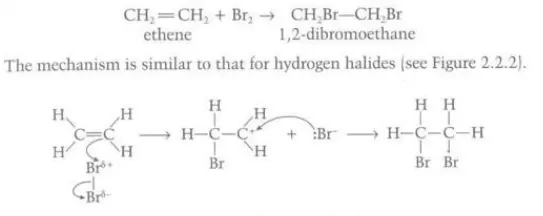
Identify this image
Alkene addition with bromine
Differentiate between the reactions of alkenes with cold and hot acidified potassium manganate (VII)
Cold acidified dilute potassium manganate (VII) turns colorless when reacting with an alkene and the alkene is converted to a diol. Hot acidified concentrated potassium manganate (VII) forms a diol and water and loses its color but immediately oxidizes to ketones, aldehydes, carboxylic acids or carbon dioxide (depending on the alkene)
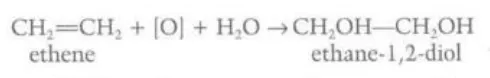
Identify this image
Cold acidified dilute potassium manganate (VII) reacting with ethene
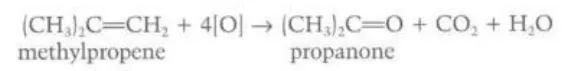
Identify this image
Hot acidified concentrated potassium manganate (VII) reacting with methylpropene
Describe the reactions between a hydrogen halide and an alkene
The electrophilic hydrogen halide attacks the double bond in the alkene and the electron pair from the double bond in the alkene readily forms a bond with the hydrogen atom due to its positive dipole which allows the halogen atom to gain control of the electron pair in the hydrogen halide to form a halide ion which attacks the carbocation to subsequently form a halogenoalkane
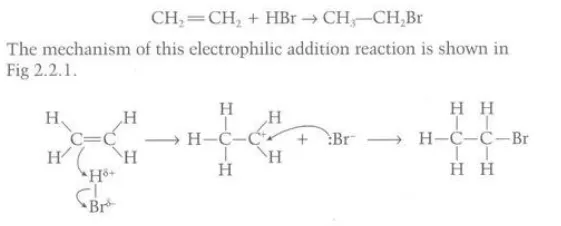
Identify this image
Addition of hydrogen halides to alkene
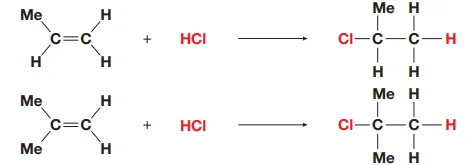
Identify this image
Markovnikov rule for addition to asymmetric alkenes
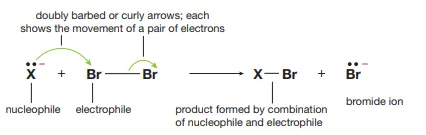
Identify this image
Electrophilic addition via heterolysis
Describe the test for identifying alkenes
When adding aqueous bromine to unsaturated compounds such as alkenes, the colour of the bromine changes from orange to colourless
State the main reactions that alcohols undergo
Oxidization by potassium permanganate (VII)/potassium dichromate, reaction with carboxylic acids and reaction with sulfuric acid
Describe the oxidization of primary, secondary and tertiary alcohols
Primary alcohols are oxidized to aldehydes and can be further oxidized to carboxylic acids. Secondary alcohols are oxidized to ketones and are not oxidized further. Tertiary alcohols cannot be oxidized without breaking a C—C bond in the alcohol
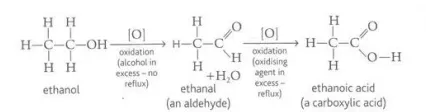
Identify this image
Oxidization of a primary alcohol to form an aldehyde which further oxidizes to form a carboxylic acid
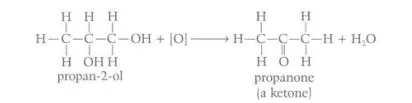
Identify this image
Oxidization of a secondary alcohol to form a ketone
Describe the conditions required to obtain aldehydes and carboxylic acids from primary alcohols
Aldehydes can only be obtained from primary alcohols if the oxidizing agent is not in excess and the acid, if present, is fairly dilute or if the aldehyde is distilled immediately.
Carboxylic acids can be obtained from primary alcohols if the oxidizing agent is added in excess or the reaction is carried out in reflux for 20 minutes
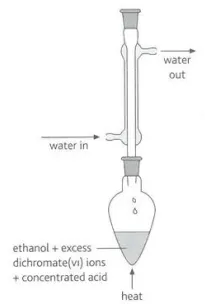
Identify this image
Primary alcohol reacting with concentrated acids in reflux to obtain the carboxylic acid
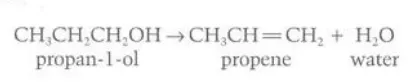
Describe the reaction of alcohols with concentrated sulfuric acid on heating
Alkene and water is formed
Describe the test for identifying secondary alcohols and ethanol
Oxidizing the CH3CHOH group with iodine in the presence of sodium hydroxide to form triiodomethane which precipitates as yellow crystals
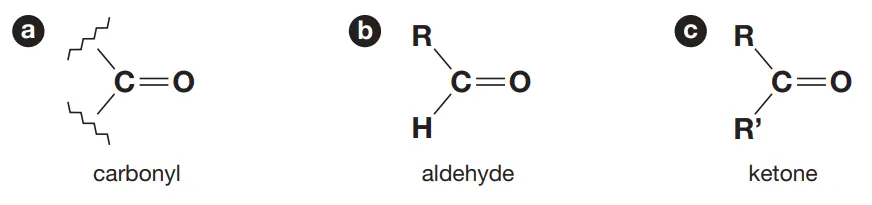
Identify this image
Difference between structure of aldehydes and ketones
Describe how Tollen’s reagent is used to distinguish between aldehydes and ketones
When aldehydes are warmed carefully with Tollen’s reagent, they are oxidized to carboxylic acids and the silver ions are reduced to silver and a ‘mirror’ is said to evolve. Ketones do not react with Tollen’s reagent
Describe how Fehling’s solution is used to distinguish between aldehydes and ketones
When aldehydes are warmed with Fehling’s solution, they are oxidized to carboxylic acid and the copper ions in the blue solution change to an orange-red precipitate of copper (I) oxide due to the copper ions oxidizing the aldehyde. Ketones do not react with Fehling’s solution
Describe how acidified potassium manganate (VI) or acidified potassium dichromate (VI) can be used to differentiate between aldehydes and ketones
The manganate oxidizes the aldehyde to carboxylic acids and the solution turns from purple to born as the manganese ions are reduced while the dichromate changes from orange to green as the chromium ions are reduced. Ketones do not react with either reagents
Describe the mechanisms behind the additions in carbonyl compounds
The high electronegativity of the oxygen atom polarizes the C=O bond and leaves the C atom open to attack by nucleophiles, such as hydrogen cyanide, which can form a negative intermediate that can react with cations, causing an addition
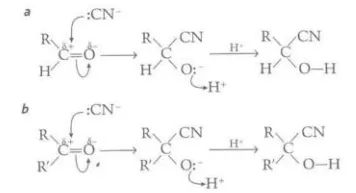
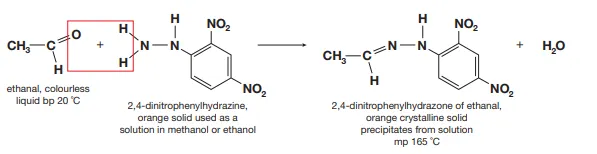
Describe the test to identify the presence of the carbonyl group and how can this be used to identify the particular aldehyde or ketone present
Brady’s reagent is added and if an aldehyde or ketone is present, a deep orange precipitate is formed. If the precipitate is purified via precipitation and the melting point is determined, we can identify the particular aldehyde or ketone present as each Brady’s reagent derivative of an aldehyde or ketone has a particular melting point
State the main reactions carboxylic acids undergo
With sodium hydroxide, metals, carbonates, alcohols and select Period 3 chlorides
Describe the reaction between sodium hydroxide and carboxylic acids
They form a sodium salt and water
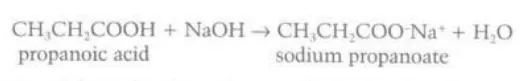
Describe reaction between carboxylic acids and metals
They form a metal salt and hydrogen
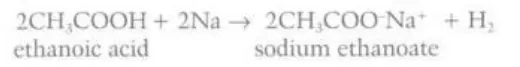
Describe the reaction between carboxylic acids and carbonates
They react to form a salt, water and carbon dioxide

Describe the reaction between carboxylic acids and alcohols
They react in the presence of an acid catalyst and heated under reflex to form volatile alcohols, esters and water
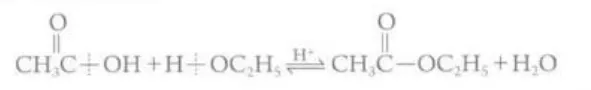
Describe the test used to identify carboxylic acids
When they are added to a carbonate, bubbles of carbon dioxide are released which turns limewater milky when bubbled through it
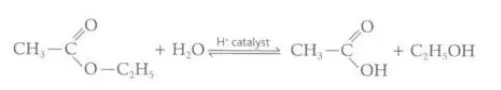
Describe how esters are broken down
They are broken down via hydrolysis by heating it under reflux with an acid or alkalic catalyst
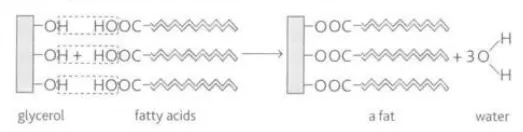
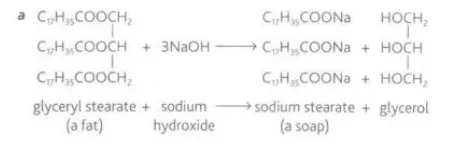
Identify this image
Reaction of fatty acids and glycerols to make fat
Identify this image
Saponification
State the conditions of transesterification
Heat and possibly a catalyst
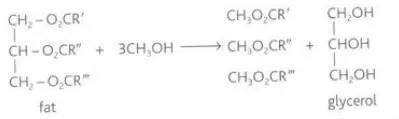
Identify this image
Transesterification
Describe how esters can be identified
Their fruity smell
Describe why amines are stronger bases than ammonia although they are both weak
The alkyl groups in amines release electrons readily and increase the electron density on the nitrogen atom
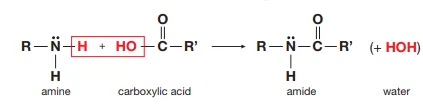
Describe the reaction of primary amines with dilute carboxylic acid
They form an amide and water
List common aromatic hydrocarbons
Benzene, phenol and their aromatic constituents
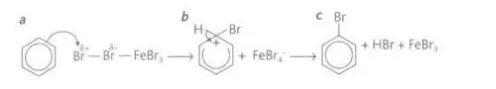
Describe the reaction of benzene with bromine
It forms bromobenzene and hydrogen bromide and it can only be done using bromide using a Lewis acid catalyst such as iron (III) bromide or under UV light
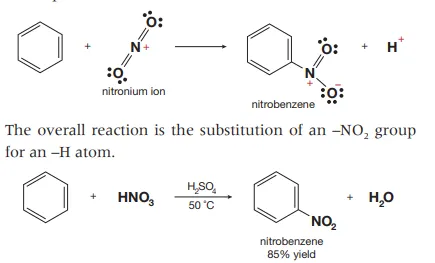
Describe the reaction between benzene and concentrate nitric acid
This is done by using sulfuric acid to add hydrogen to the oxygen bonded to the hydrogen in the acid which produces the nitronium ion through polarizing which acts as an electrophile that attacks the delocalized electrons in the benzene ring which is then added to form nitrobenzene.
Describe the reaction between methylbenzene and bromine
They react to form a mixture of two isomers of bromotoulene as the bromine atom can either subsitute the 2C (ortho) or 4C (para position) on the atomic ring. In addition, hydrogen bromide tends to form as some of the displaced hydrogen atoms can form with the bromine

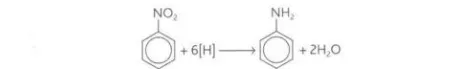
Describe the reaction between nitrobenzene, tin and concentrated HCl
It forms phenylamine and water
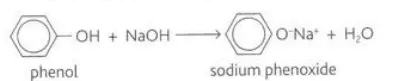
Describe the reaction between phenol and sodium hydroxide
They react due to the alcoholic nature of phenol to form sodium phenoxide and water
Describe the reaction between phenol and acyl halides
They form esters and hydrogen chloride gas
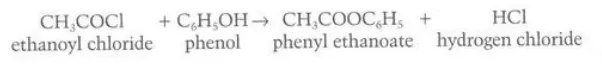
Describe the reaction between bromine water and phenol
A lone pair of electrons on the oxygen atoms of the phenol overlap with its delocalized electrons in its ring which increases its electron density allowing stable intermediates to be formed when bromine is introduced to phenol, forming a white precipitate of 2,4,6,-tribromophenol without a halogen carrier
Describe diazotisation
For the reaction, a stable version of nitrous acid is created by adding hydrochloric acid to sodium nitrite. Phenylamine reacts with the nitrous acid and the mixture is kept below ten degrees Celsius to prevent the diazonium salt from decomposing to nitrogen.
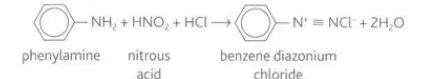
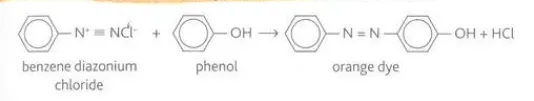
Describe the reaction between benzenediazonium chloride and phenol
They react to form an orange dye via a coupling reaction and the orange azo dye is kept below 10 degrees Celsius to prevent decomposition