The Periodic table: 11.1 to 11.4 COMPLETE
Elements are arranged on the Periodic Table in order of increasing atomic number, where each element has one proton more than the element preceding it
The table is arranged in vertical columns called Groupsnumbered I - VIII (numbers shown on the course's Periodic Table) and in rows called Periods (numbers not shown on the Periodic Table)
Period: these are the horizontal rows that show the number of shells of electrons an atom has
- Eg elements in Period 2 have two electron shells, elements in Period 3 have three electron shells
Group: these are the vertical columns that show how many outer electrons each atom has
- Eg Group 4/ IV elements have atoms with 4 electrons in the outermost shell, Group 6 elements have atoms with 6 electrons in the outermost shell


The Properties of The Periodic Table
Because there are patterns in the way the elements are arranged on the Periodic table, there are also patterns and trends in the chemical behaviour of the elements and their physical properites
There are trends in properties down Groups and across each Period
All of the Group I elements, for example, react very quickly with water
In this way the Periodic Table can be used to predict how a particular element will behave
The Periodic Table can also be used to predict boiling point, melting point, density and many more properties by comparing to nearby elements
11.2 Group 1 The Alkali metals
- The metallic character of the elements decreases as you move across a Period on the Periodic Table, from left toright, and it increases as you move down a Group
- This trend occurs due to atoms more readily acceptingelectrons to fill their valence shells rather than losing them to have the previous, already full, electron shell as their outer shell
- Metals occur on the left-hand side of the Periodic Table and non-metals on the right-hand side
- Between the metals and the non-metals lie the elements which display some properties of both
- These elements are referred to as metalloids or semi-metals
Properties of Metals and Non metals
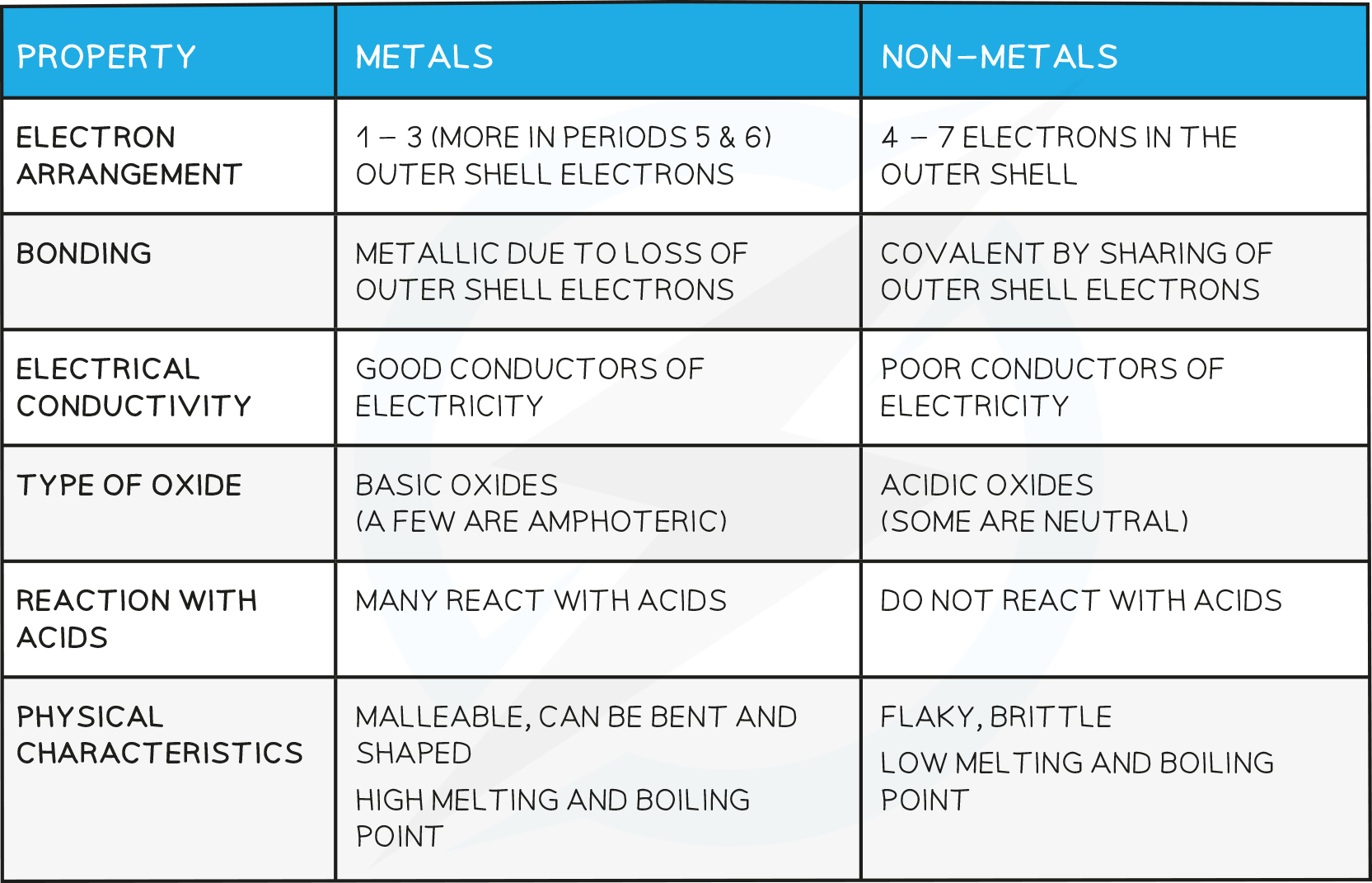

Electronic Configuration:
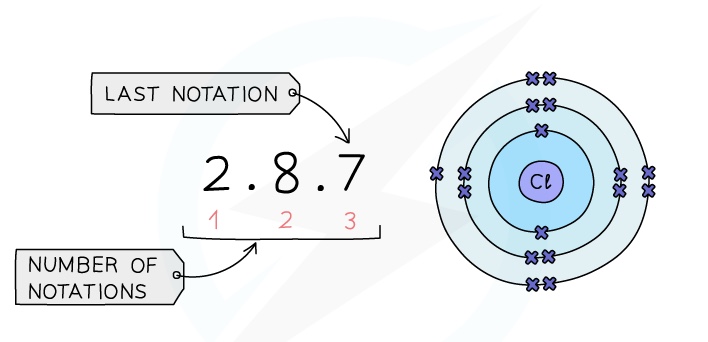
- The electronic configuration is the arrangement of electrons into shells for an atom (e.g: the electronic configuration of carbon is 2,4)
- There is a link between the electronic configuration of the elements and their position on the Periodic Table
- The number of notations in the electronic configuration will show the number of occupied shells of electrons the atom has, showing the period
- The last notation shows the number of outer electrons the atom has, showing the group number
Example: Electronic configuration of chlorine:
Chemical Properties of the elements in the same group:
- Elements in the same group in the Periodic Table have similar chemical properties
- When atoms collide and react, it is the outermost electrons that interact
- The similarity in their chemical properties stems from having the same number of electrons in their outer shell
- For example, both lithium and sodium are in Group 1 and can react with elements in Group 7 to form an ionic compound (charges of Group 1 ions are 1+, charges of Group 7 ions are 1-) by reacting in a similar manner and each donating one electron to the Group 7 element
- As you look down a group, a full shell of electrons is added to each subsequent element
- Lithium's electronic configuration: 2,1
- Sodium's electronic configuration: 2,8,1
- Potassium's electronic configuration: 2,8,8,1
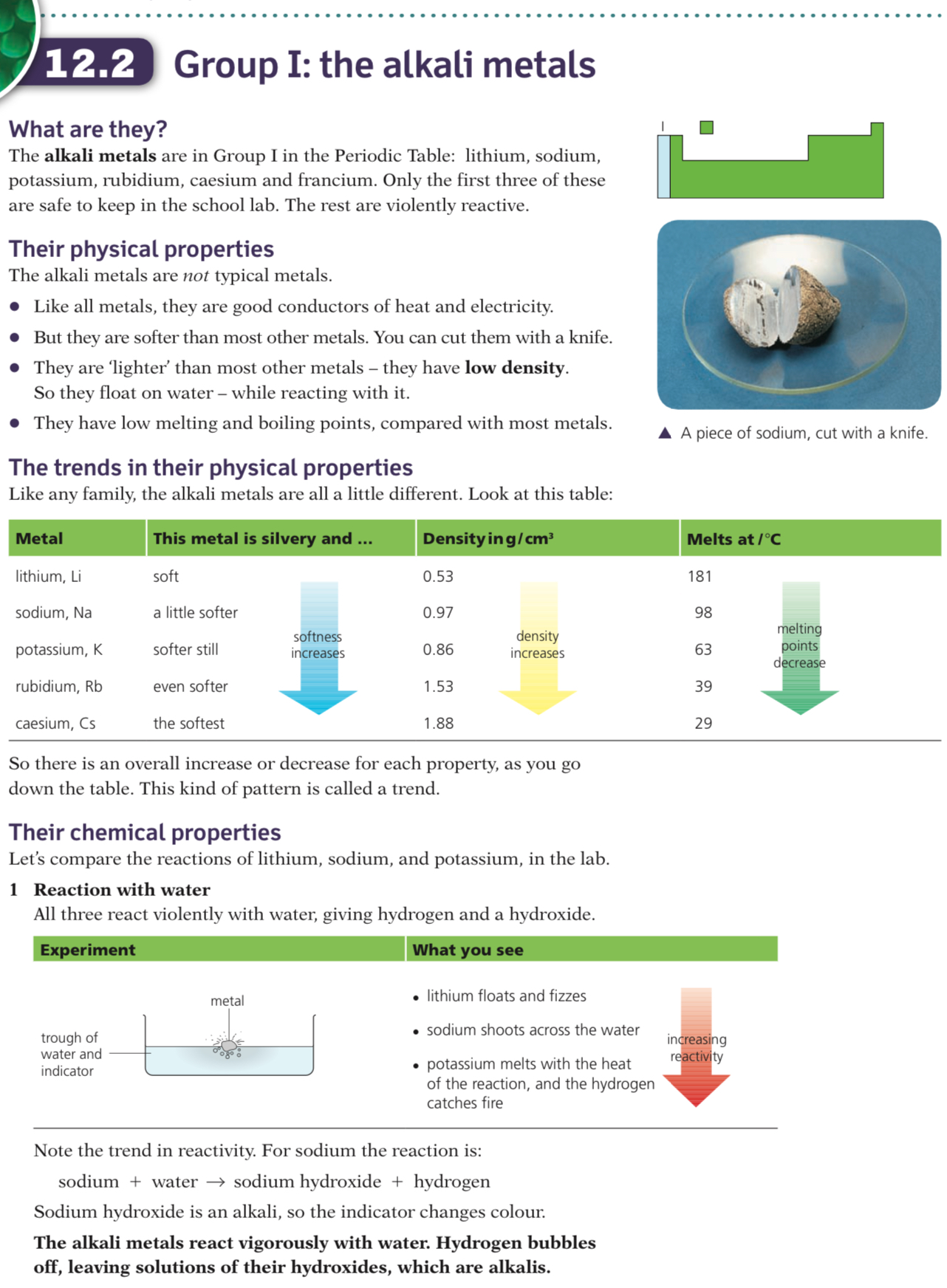
Properties of the Group 1 Metals
- The Group I metals are also called the alkali metals as they form alkaline solutions with high pH values when reacted with water
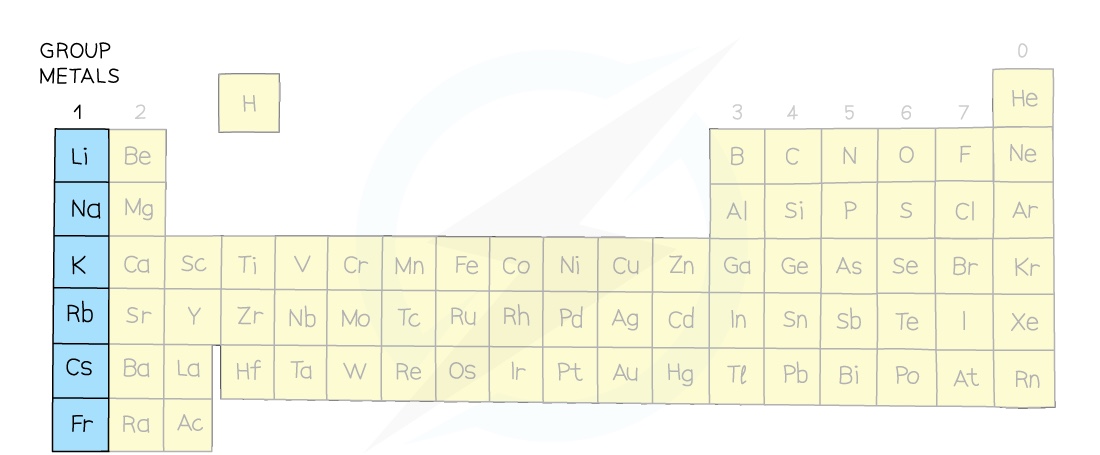
- Group I metals are lithium, sodium, potassium, rubidium, caesium and francium
- They all contain just one electron in their outer shell
Physical properties of the Group I metals
The Group I metals:
- Are soft and easy to cut, getting even softer and denser as you move down the Group (sodium and potassium do not follow the trend in density)
- Have shiny silvery surfaces when freshly cut
- Conduct heat and electricity
- They all have low melting points and low densities compared to other metals, and the melting point decreases as you move down the Group; some would melt on a hot day
Chemical Properties
They react readily with oxygen and water vapour in air so they are stored under oil to stop them from reacting
Group I metals will react similarly with water, reacting vigorously to produce an alkaline metal hydroxide solution and hydrogen gas
The Group I metals get more reactive as you look down the group, so only the first three metals are allowed in schools for demonstrations

The reactivity of the ^^Group 1 metals increases as you go down the group^^
Each outer shell contains only one electron so when they react, they lose the outer electron which empties the outermost shell
The next shell down automatically becomes the outermost shell and is already full, hence the atom obtains an electronic configuration which has a full outer shell of electrons and is stable
As you go down Group 1, the number of inner complete shells of electrons increases by 1 per row (period number increases down the Periodic Table)
This means that the outer electron is further away from the nucleus so there are weaker electrostatic forces of attraction
This requires less energy to overcome the electrostatic forces of attraction between the negatively charged electron and the positively charged nucleus
This allows the electron to be lost more easily, making the Group I metal atoms more reactive as you go down the group
Properties of other Alkali Metals (Rubidium, Caesium and Francium)
- As the reactivity of alkali metals increases down the group, rubidium, caesium and francium will react more vigorously with air and water than lithium, sodium and potassium
- Lithium will be the least reactive metal in the group at the top, and francium will be the most reactive at the bottom
- Francium is rare and radioactive so is difficult to confirm predictions
11.3 Group VII: The Halogens Properties
A non metal group
These are the Group VII non-metals that are poisonousand include fluorine, chlorine, bromine, iodine and astatine
Halogens are diatomic, meaning they form molecules of two atoms
All halogens have seven electrons in their outer shell
They form halide ions by gaining one more electron to complete their outer shells
Fluorine is not allowed in schools so observations and experiments tend to only involve chlorine, bromine and iodine
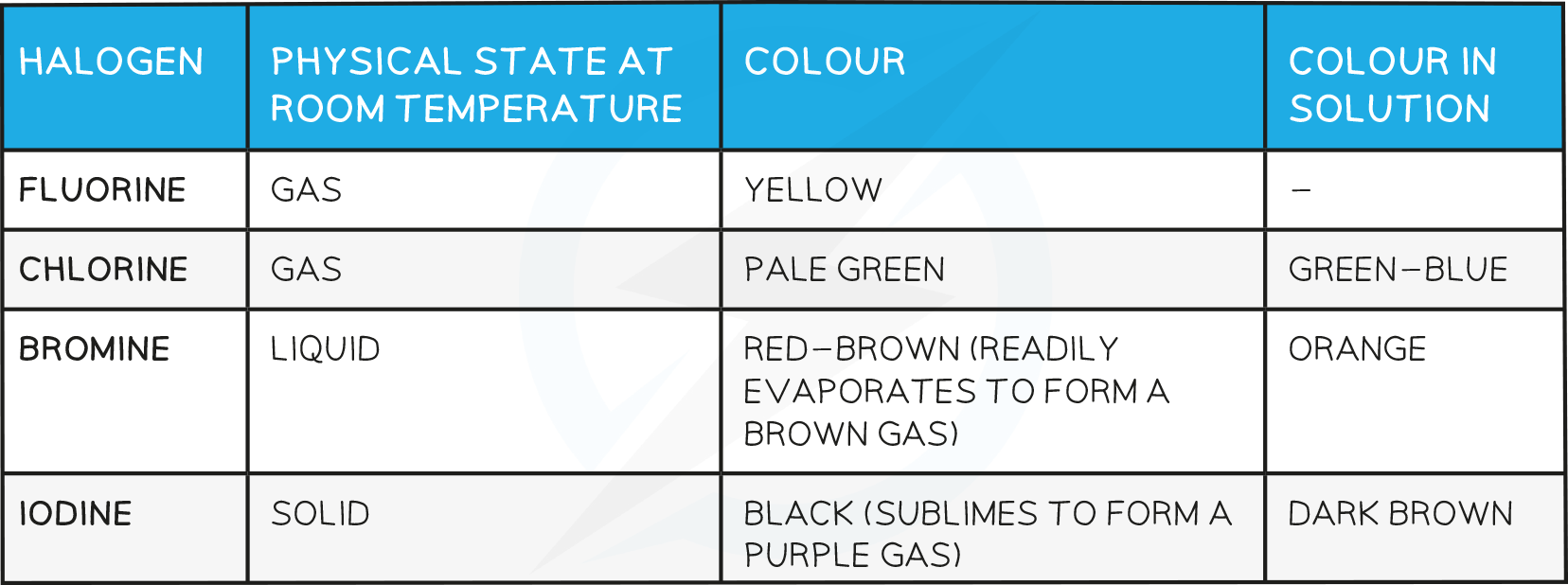
Physical factors eg Melting Point
- The density and melting and boiling points of the halogens increase as you go down the group
State at room temperature
At room temperature (20 °C), the physical state of the halogens changes as you go down the group
Fluorine and chlorine are gases, bromine is a liquid and iodine is a solid
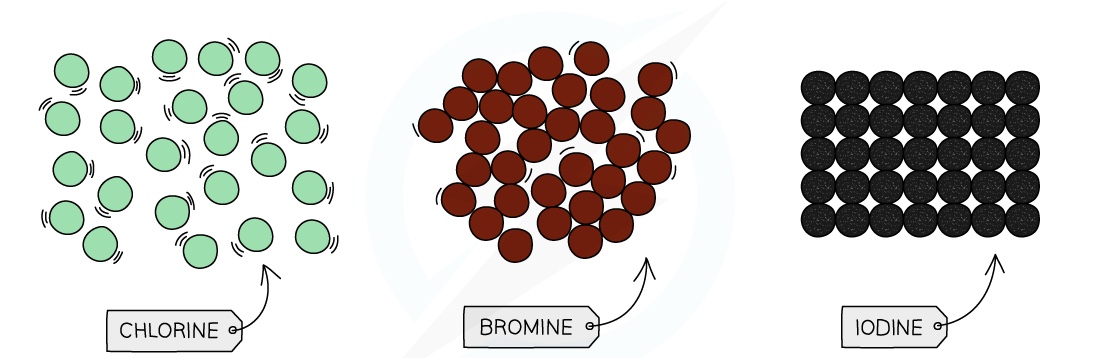
Colour
The halogens become darker as you go down the group
Chlorine is pale %%green%%, bromine is ==red-brown== and iodine is black

Electronic Configuration:
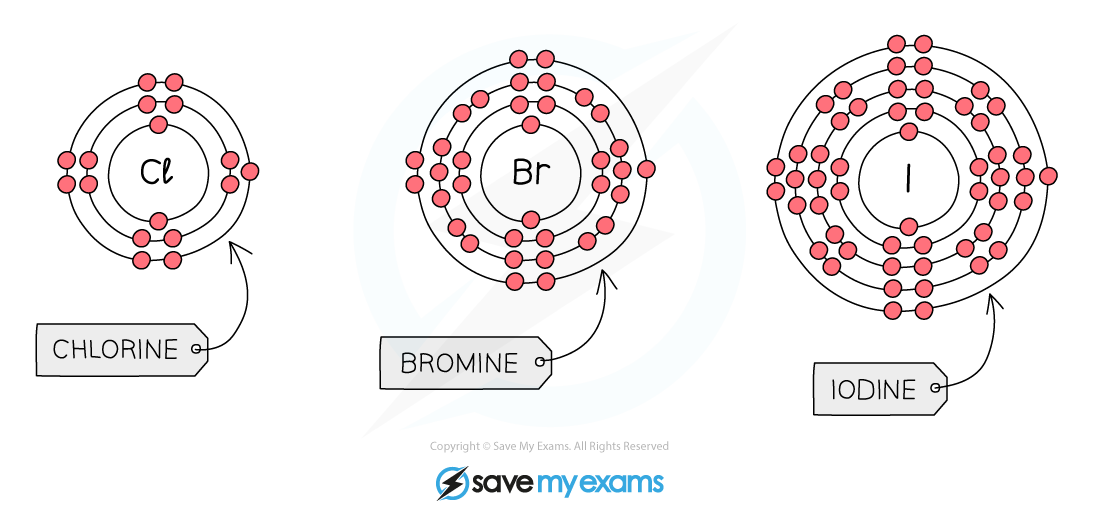
The Trend in Reactivity:
- Reactivity of Group VII non-metals increases as you go upthe group (this is the opposite trend to that of Group I)
- Each outer shell contains seven electrons and when the halogen reacts, it will need to gain one outer electron to get a full outer shell of electrons
- As you go up Group VII, the number of shells of electrons decreases (period number decreases moving up the Periodic Table)
- This means that the outer electrons are closer to the nucleus so there are stronger electrostatic forces of attraction, which help to attract the extra electron needed
This allows an electron to be attracted more readily, so the higher up the element is in Group VII then the morereactive it is
Reaction of Halogens with Halide ions in displacement reactions
A halogen displacement reaction occurs when a more reactive halogen displaces a less reactive halogen from an aqueous solution of its halide
The reactivity of Group VII non-metals increases as you move up the group
Out of the three commonly used halogens, chlorine, bromine and iodine, chlorine is the most reactive and iodine is the least reactive

Halogen Displacement reactions
%%Chlorine and Bromine%%
- If you add chlorine solution to colourless potassium bromide solution, the solution becomes orange as bromine is formed
- Chlorine is above bromine in Group VII so is more reactive
- Chlorine will therefore displace bromine from an aqueous solution of metal bromide
- The least reactive halogen always ends up in elemental form
potassium bromide + chlorine → potassium chloride + bromine
2KBr (aq) + Cl2 (aq) → 2KCl (aq) + Br2 (aq)
Bromine and iodine
- Bromine is above iodine in Group VII so is more reactive
- Bromine will therefore displace iodine from an aqueous solution of metal iodide
magnesium iodide + bromine → magnesium bromide + iodine
2MgI (aq) + Br2 (aq) → 2MgBr (aq) + I2 (aq)
Properties of Other Halogens (Fluorine and Astatine
Melting and Boiling points
- The melting and boiling point of the halogens increases as you go down the group
- Fluorine is at the top of Group VII so will have the lowestmelting and boiling point
- Astatine is at the bottom of Group VII so will have the highest melting and boiling point
Physical states
- The halogens become denser as you go down the group
- Fluorine is at the top of Group VII so will be a gas
- Astatine is at the bottom of Group VII so will be a solid
Colour
The colour of the halogens becomes darker as you go down the group
Fluorine is at the top of Group VII so the colour will be lighter, so fluorine is yellow
Astatine is at the bottom of Group VII so the colour will be darker, so astatine is black


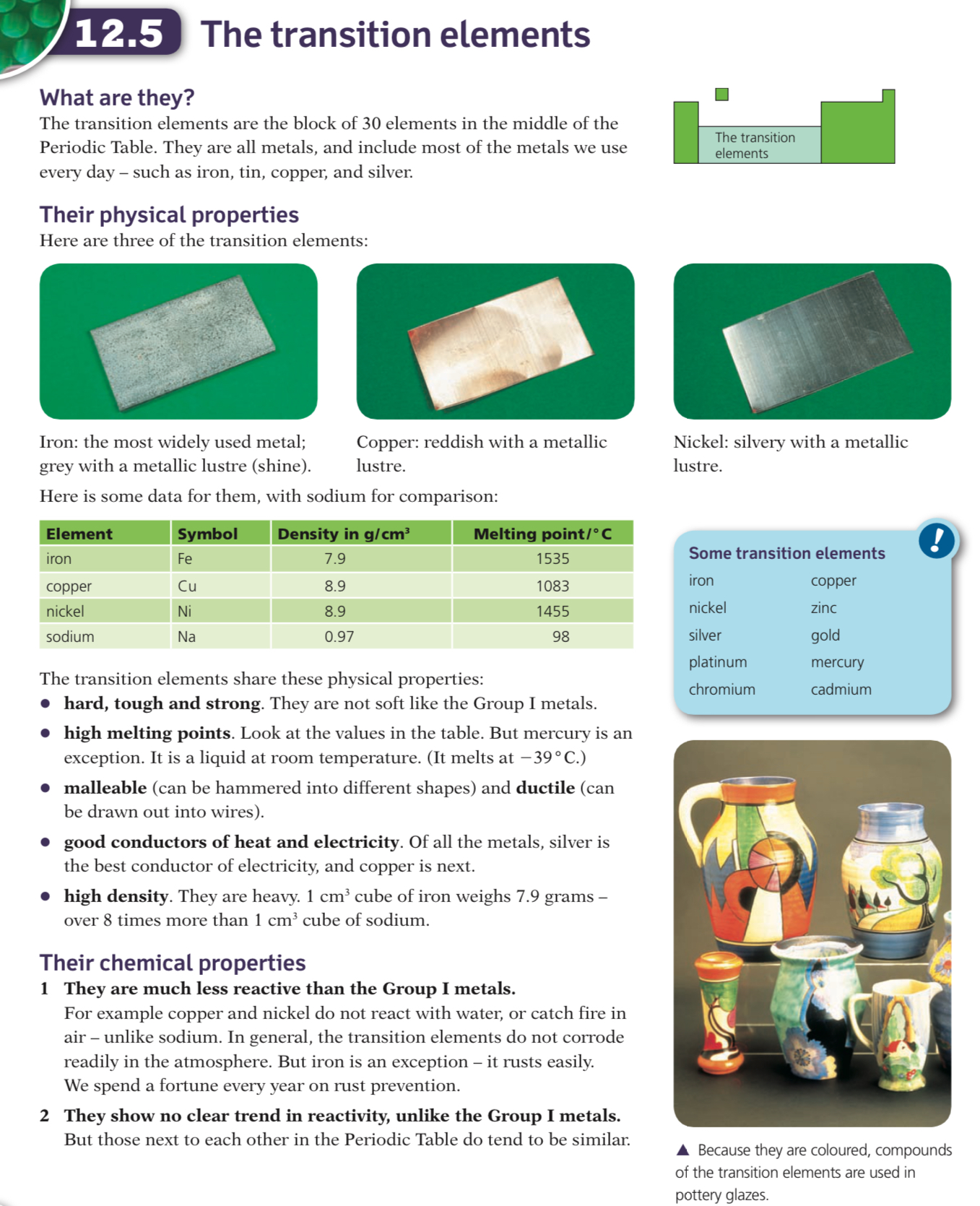
__11.4 Transition elements __
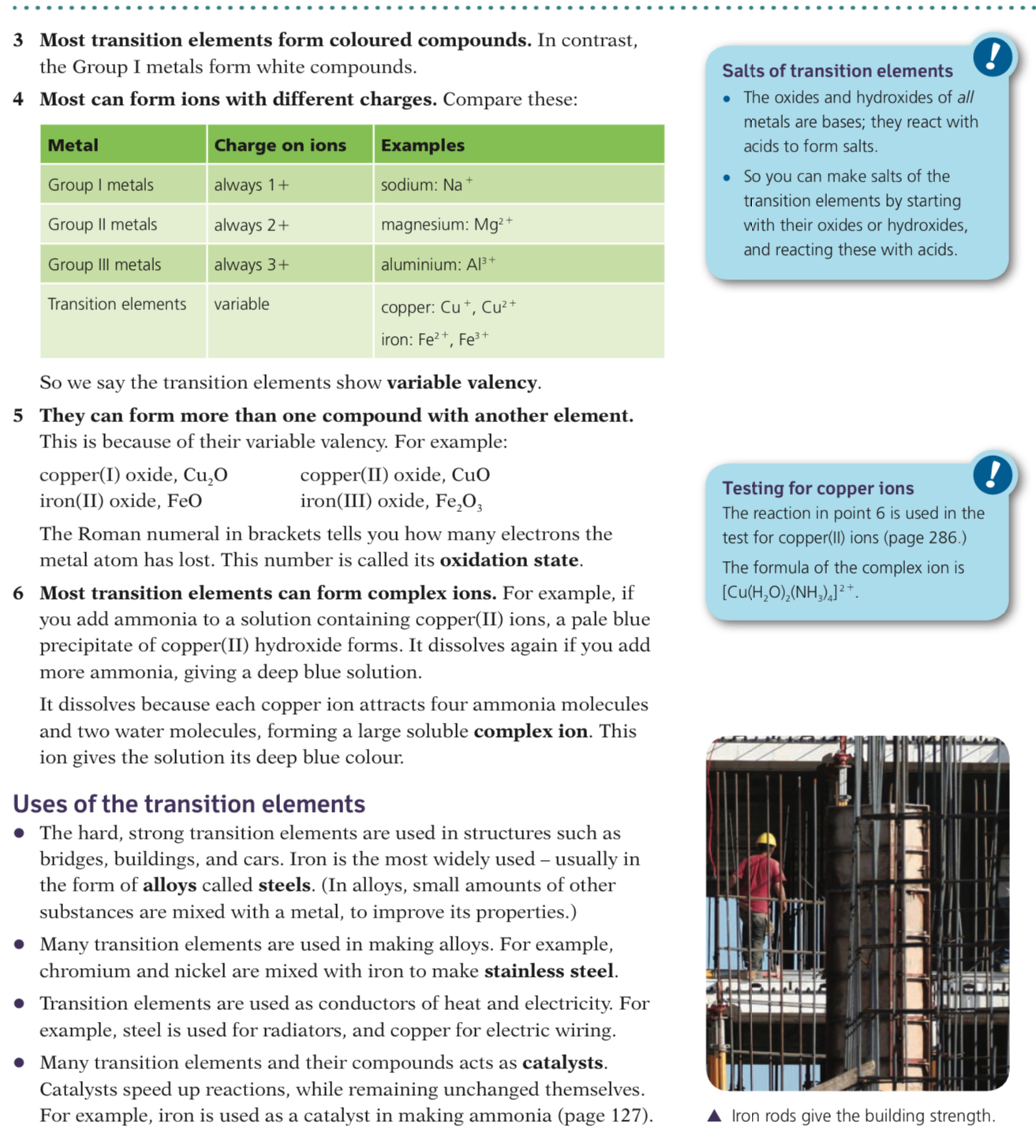
They are very hard and strong metals and are good conductors of heat and electricity
They have very high melting points and are highly densemetals
For example, the melting point of titanium is 1,688ºC whereas potassium in Group I melts at only 63.5ºC, slightly warmer than the average cup of hot chocolate!
The transition elements form coloured compounds and often have more than one oxidation state, such as iron readily forming compound of both Fe2+ and Fe3+
These coloured compounds are responsible for the pigments in many paints and the colours of gemstones and rocks
Transition elements, as elements or in compounds, are often used as catalysts to improve the rate or reaction in industrial processes
- Transition element catalysts of platinum or rhodium are also used in car exhausts in the 'catalytic convertor' to reduce the levels of nitrous oxides and carbon monoxide produced

Summing up:
1- Form colored compounds
2- used as catalysts
3- high melting points
4- good conductors of heat and electricity
Variable Oxidation states
The transition elements have more than one oxidation state, as they can lose a different number of electrons, depending on the chemical environment they are in
Iron for example can lose two electrons to form Fe2+ or three electrons to form Fe3+
Compounds containing transition elements in different oxidation states will have different properties and colours
Extended IGCSE the uses of Transition Elements
- The transition elements are used extensively as catalystsdue to their ability to interchange between a range of oxidation states
- This allows them to form complexes with reagents which can easily donate and accept electrons from other chemical species within a reaction system
- They are used in medicine and surgical applications such as limb and joint replacement (titanium is often used for this as it can bond with bones due to its high biocompatibility)
- They are also used to form coloured compounds in dyesand paints, stained glass jewelry
11.5 The Nobel Gases
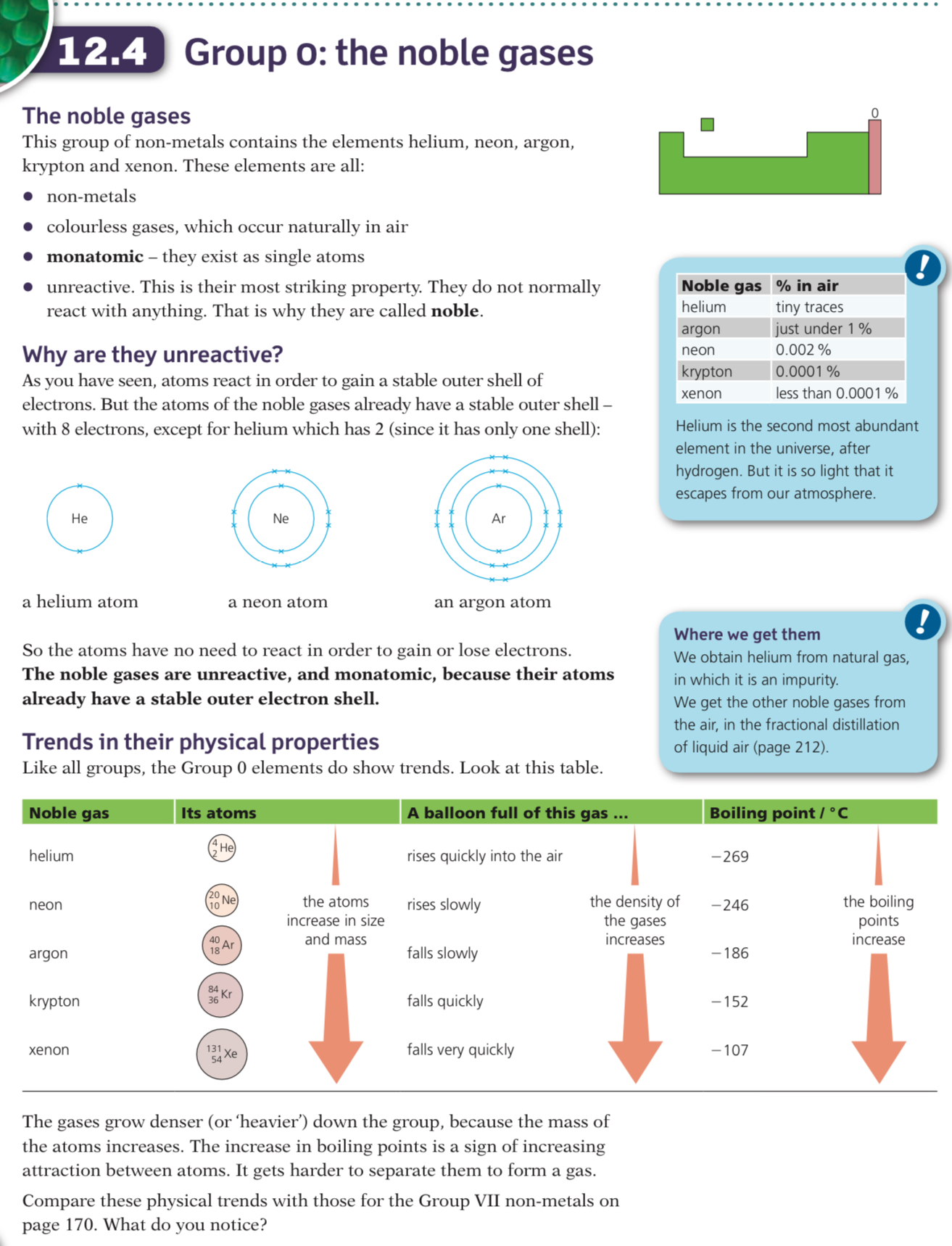
The noble gases are in Group VIII (or Group 0); they are non-metals and have very low melting and boiling points
They are all monoatomic, colourless gases
The Group 0 elements all have full outer shells
This electronic configuration is extremely stable so these elements are unreactive and are inert
Electronic configurations of the noble gases:
- He: 2
- Ne: 2,8
- Ar: 2,8,8
- Kr: 2,8,18,8
- Xe: 2,8,18,18,8

Uses of Nobel Gases
Helium is used for filling balloons and weather balloons as it is less dense than air and does not burn
Neon, argon and xenon are used in advertising signs
Argon is used to provide an inert atmosphere for welding
Argon is also used to fill electric light bulbs as it is inert
Neon and argon are used as inert atmospheres for sensitive experiments where nitrogen is not appropriate

\n \n
\n \n
\n \n
\n \n